Andrew E. Sloan, Keith A. Black, and Donald P. Becker
The treatment of sellar lesions is one of the more challenging and satisfying aspects of skull base surgery. The wide variety of lesions that occur in this region and the complexity of their treatment necessitates a thorough understanding of sellar anatomy, as well as the clinical symptoms and diagnostic evaluation of the myriad disease processes involving the pituitary gland.
ANATOMY
Sphenoid Bone and Sella Turcica
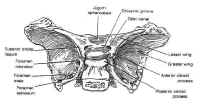
 The sphenoid bone is located at the base of the skull, posteroinferior to the anterior cranial fossa and anterior to the temporal and occipital bones (Fig. 1). The sella itself is a midline depression in the mid-portion of the sphenoid bone. It is bounded anteriorly and inferiorly by the sellar floor, anterolaterally by the anterior clinoid process, laterally by the cavernous sinuses, posteriorly by the dorsum sellae and the posterior clinoid processes, and superiorly by the diaphragmasellae. The floor of the sella is lined with an endocranial layer that is continuous with the diaphragma sellae superiorly, and contains the intercavernous or circular sinuses. These are highly variable, but typically develop in the anterosuperior and posterosuperior regions of the sella. The cavernous sinuses drain into the basilar venous plexus posterior to the dorsum sellae along the upper clivus, which joins the superior and inferiorpetrosal sinuses.
The sphenoid bone is located at the base of the skull, posteroinferior to the anterior cranial fossa and anterior to the temporal and occipital bones (Fig. 1). The sella itself is a midline depression in the mid-portion of the sphenoid bone. It is bounded anteriorly and inferiorly by the sellar floor, anterolaterally by the anterior clinoid process, laterally by the cavernous sinuses, posteriorly by the dorsum sellae and the posterior clinoid processes, and superiorly by the diaphragmasellae. The floor of the sella is lined with an endocranial layer that is continuous with the diaphragma sellae superiorly, and contains the intercavernous or circular sinuses. These are highly variable, but typically develop in the anterosuperior and posterosuperior regions of the sella. The cavernous sinuses drain into the basilar venous plexus posterior to the dorsum sellae along the upper clivus, which joins the superior and inferiorpetrosal sinuses.
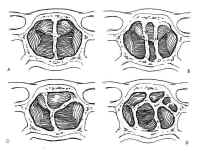
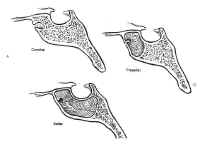 The sphenoid sinus is just anterior and inferior to the sella, and is divided by one or more vertical septa that are often asymmetric (Fig.2). The degree of pneumatization of the sphenoid sinus varies among individuals and is classified as conchal, parasellar, or sellar (1,2) (Fig. 3). The sellar type is well pneumatized beneath the entire sella, which bulges into the sinus and is found in 70% to 86% of adults. The presellar sphenoid sinus extends only to the mid-portion of the sella, has no sphenoid bulges, and is found in 11% to 24% of adults. The conchal sphenoid sinus has minimal pneumatization andat least 10 mm of bone between the undeveloped sphenoid sinus and the sella turcica. This configuration is common in prepubertal children, but occurs in only 3% of adults.
The sphenoid sinus is just anterior and inferior to the sella, and is divided by one or more vertical septa that are often asymmetric (Fig.2). The degree of pneumatization of the sphenoid sinus varies among individuals and is classified as conchal, parasellar, or sellar (1,2) (Fig. 3). The sellar type is well pneumatized beneath the entire sella, which bulges into the sinus and is found in 70% to 86% of adults. The presellar sphenoid sinus extends only to the mid-portion of the sella, has no sphenoid bulges, and is found in 11% to 24% of adults. The conchal sphenoid sinus has minimal pneumatization andat least 10 mm of bone between the undeveloped sphenoid sinus and the sella turcica. This configuration is common in prepubertal children, but occurs in only 3% of adults.
Nasal Cavity
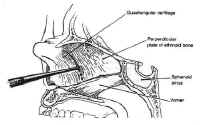 The nasal cavity provides access to the sphenoid sinus for transsphenoidal approaches. The most important anatomic features for the surgeon are the medial structures comprising the anterior septum that support the nose. These consist of the septal cartilage ventrally and superiorly, the vomer posteriorly and inferiorly, and the perpendicular plate of the ethmoid (Fig. 4).
The nasal cavity provides access to the sphenoid sinus for transsphenoidal approaches. The most important anatomic features for the surgeon are the medial structures comprising the anterior septum that support the nose. These consist of the septal cartilage ventrally and superiorly, the vomer posteriorly and inferiorly, and the perpendicular plate of the ethmoid (Fig. 4).
Pituitary Gland and Hypothalamus
The pituitary gland resides in the sella turcica and is attached tothe hypothalamus by the pituitary stalk. The stalk enters thesella through an opening in the diaphragma sellae, a membrane ofbasilar dura stretched between the tuberculum sellae and theposterior clinoids that separates the cranium from the sella. Asmall outpouching of the arachnoid accompanies the stalk throughthe central opening in the diaphragma in about 50% of specimens,to form a small pituitary cistern anterosuperior to theadenohypophysis (3). This is a potential source of cerebrospinalfluid (CSF) leak in the transsphenoidal approach to sellarlesions.
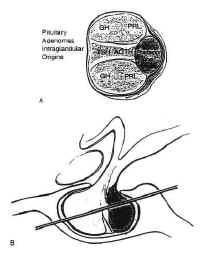 Figure 5The pituitary is composed of two lobes,distinct in embryologic origin, structure, and function, whichcome together during embryogenesis. The adenohypophysis isyellowish in color, and has a relatively firm consistency. Itarises from the primitive stomodeum as Rathkes pouch, whichgrows toward the neural tube to form the craniopharyngeal duct.The anterior portion of Rahtkes pouch develops into the parsdistalis, the functional portion of the anterior pituitary. A thinsleeve of tissue derived from the adenohypophysis extends upwardalong the pituitary stalk to form the pars tuberalis, which risesa short distance above the diaphragma sellae. The posterior wallof Rathkes pouch develops into the intermediate lobe, a cysticstructure, vestigial in function, lined by ciliated,mucus-producing adenohypophyseal cells. Although theadenohypophysis is uniform in structure, the cells aredifferentially concentrated in three regions. The cells in thecentral “mucoid wedge” produce thyroid-stimulatinghormone (TSH) anteriorly and adrenocorticotropic hormone (ACTH)posteriorly, and are basophilic (Fig. 5).
Figure 5The pituitary is composed of two lobes,distinct in embryologic origin, structure, and function, whichcome together during embryogenesis. The adenohypophysis isyellowish in color, and has a relatively firm consistency. Itarises from the primitive stomodeum as Rathkes pouch, whichgrows toward the neural tube to form the craniopharyngeal duct.The anterior portion of Rahtkes pouch develops into the parsdistalis, the functional portion of the anterior pituitary. A thinsleeve of tissue derived from the adenohypophysis extends upwardalong the pituitary stalk to form the pars tuberalis, which risesa short distance above the diaphragma sellae. The posterior wallof Rathkes pouch develops into the intermediate lobe, a cysticstructure, vestigial in function, lined by ciliated,mucus-producing adenohypophyseal cells. Although theadenohypophysis is uniform in structure, the cells aredifferentially concentrated in three regions. The cells in thecentral “mucoid wedge” produce thyroid-stimulatinghormone (TSH) anteriorly and adrenocorticotropic hormone (ACTH)posteriorly, and are basophilic (Fig. 5).
Some of these ACTH-producing cells invaginatethe posterior pituitary with increasing age, a phenomenon known as”basophilic invasion.” The cells of the lateral “acidophilicwings” produce primarily the peptide growth hormoneanteriorly and prolactin posteriorly. Gonadotropic cells producingluteinizing hormone and follicle-stimulating hormone are locateddiffusely throughout the gland.
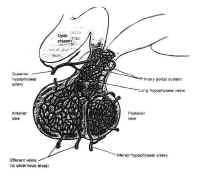 Figure 6The adenohypophysis is vascularizedpredominately by the superior hypophyseal artery by way of thehypophyseal portal system (Fig. 6). This venous plexus originatesin the anterior inferior portion of the hypothalamus and the floorof the third ventricle, where capillaries with a specializedfenestrated epithelium arise from branches of the superiorhypophyseal arteries. These capillaries are associated with theterminals of the tuberoinfundibular neurons, which synthesize thehypothalamic-releasing hormones and secrete them into thecapillary network. These vessels drain into the hypothalamicportal veins, which descend to the adenohypophysis along theanterior portion of the stalk, giving it a striated appearance. Inthe adenohypophysis, the portal veins develop into a secondcapillary network that supplies the secretory cells of theadenohypophysis and drains into the cavernous sinus.
Figure 6The adenohypophysis is vascularizedpredominately by the superior hypophyseal artery by way of thehypophyseal portal system (Fig. 6). This venous plexus originatesin the anterior inferior portion of the hypothalamus and the floorof the third ventricle, where capillaries with a specializedfenestrated epithelium arise from branches of the superiorhypophyseal arteries. These capillaries are associated with theterminals of the tuberoinfundibular neurons, which synthesize thehypothalamic-releasing hormones and secrete them into thecapillary network. These vessels drain into the hypothalamicportal veins, which descend to the adenohypophysis along theanterior portion of the stalk, giving it a striated appearance. Inthe adenohypophysis, the portal veins develop into a secondcapillary network that supplies the secretory cells of theadenohypophysis and drains into the cavernous sinus.
The neurohypophysis, or posterior lobe, appears gray in color and hasa soft, gelatinous consistency. It arises from the medianeminence, a downpouching in the floor of the third ventricle. Theupper portion becomes the pituitary stalk, whereas the distal endfuses anteriorly with the adenohypopjysis and remnants of thecraniopharyngeal duct and becomes the neurohypophysis. The stalkcomprises unmyelinated axons from the tuberoinfundibularneurosecretory neurons of the supraoptic and paraventricularnuclei of the hypothalamus, which transport granules ofvasopressin and oxytocin synthesized by these neurons to theposterior pituitary. These granules are stored in the nerveterminals of the neurohypophysis until released by actionpotentials generated in the cell bodies of the hypothalamicnuclei. The neurohypophysis is vascularized primarily by theinferior hypophyseal arteries, although the portal vessels alsoform some anastomoses with capillaries of the neurohypophysis.
Parasellar Structures
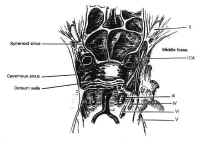 Figure 7Parasellar structures include the optic nerve and chiasm; the internalcarotid artery and its branches; the cavernous sinus and thecranial nerves contained within it; and the medial temporal lobes(Fig. 7). Symptoms and signs of sellar and parasellar lesions areoften the result of involvement of these structures. The opticchiasm overlies the diaphragm in about 80% of people. Theremainder are equally split between prefixed chiasms overlying thetuberculum sellae and postfixed chiasms overlying the dorsumsellae. The cavernous sinuses are lateral to the sella, andcontain the carotid artery and the sixth cranial nerve suspendedby fibrous trabecula. The third and fourth cranial nerves, and theophthalmic and maxillary divisions of the trigeminal nerve, liewithin the lateral walls of the cavernous sinus.
Figure 7Parasellar structures include the optic nerve and chiasm; the internalcarotid artery and its branches; the cavernous sinus and thecranial nerves contained within it; and the medial temporal lobes(Fig. 7). Symptoms and signs of sellar and parasellar lesions areoften the result of involvement of these structures. The opticchiasm overlies the diaphragm in about 80% of people. Theremainder are equally split between prefixed chiasms overlying thetuberculum sellae and postfixed chiasms overlying the dorsumsellae. The cavernous sinuses are lateral to the sella, andcontain the carotid artery and the sixth cranial nerve suspendedby fibrous trabecula. The third and fourth cranial nerves, and theophthalmic and maxillary divisions of the trigeminal nerve, liewithin the lateral walls of the cavernous sinus.
| Table 1. Differential diagnosis of sellar and parasellar masses | |
| Neoplastic lesions | Nonneoplastic lesions |
| Pituitary neoplasms | Cysts |
| Pituitary adenomas | Rathkes cleft cysts |
| Pituitary carcinomas | Mucoceles |
| Granular cell tumors | Arachnoid cysts |
| Nonpituitary neoplasms | Inflammatory and infections lesions |
| Craniopharyngiomas | Lympyhocytic hypophysitis |
| Meningiomas | Sarcoidosis |
| Chordomas | Giant cell granulomas |
| Dermoid cysts | Langerhans cell histiocytosis (histiocytosis X) |
| Epidermoid cysts | Abscesses |
| Germinomas | Vascular lesions |
| Teratomas | Aneurysms |
| Lipomas | Carotid cavernous fistulas |
| Melanomas | Cavernous angiomas |
| Metastases | |
PATHOLOGY
The close apposition of neural, endocrine, vascular, and meningeal tissue in the confined region of the sella gives rise to a large number of pathologic entities. Many of these are listed in Table 1. A complete pathologic description of each of these lesions, many of which are rare, is beyond the scope of this chapter. The pituitary adenoma and the craniopharyngioma, the most common sellar lesions, are discussed in detail. Other common neoplastic, cystic, inflammatory and infectious lesions are addressed only briefly.
Neoplastic Lesions
Pituitary Adenomas and Carcinomas
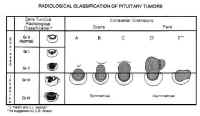 Figure 8Pituitary adenomas are the most common sellar neoplasm, accounting for 15% of all intracranial tumors. The annual incidence of these lesions is about 15 per 100,000 population (4). It is likely, however, that these symptomatic adenomas represent only a fraction of the true incidence of these neoplasms, because their prevalence in unselected autopsies is 22.5% (5). The highest incidence occurs in the third to sixth decades of life. Statistically, there appears to be an increased incidence in premenopausal women, though many contend that this is merely because of the sensitivity of the menstrual cycle to the relatively minimal endocrinologic imbalances often produced by these neoplasms. The symptoms produced in men are far less conspicuous and often ignored.
Figure 8Pituitary adenomas are the most common sellar neoplasm, accounting for 15% of all intracranial tumors. The annual incidence of these lesions is about 15 per 100,000 population (4). It is likely, however, that these symptomatic adenomas represent only a fraction of the true incidence of these neoplasms, because their prevalence in unselected autopsies is 22.5% (5). The highest incidence occurs in the third to sixth decades of life. Statistically, there appears to be an increased incidence in premenopausal women, though many contend that this is merely because of the sensitivity of the menstrual cycle to the relatively minimal endocrinologic imbalances often produced by these neoplasms. The symptoms produced in men are far less conspicuous and often ignored.
Pituitary adenomas have been classified by radiologic appearance, clinical presentation, and pathologic characteristics. The radiologic classification, known as the Hardy classification, distinguishes adenomas based on size and gross pathoanatomic relationships (6) (Fig. 8: Table 2). Adenomas 10 mm or less in diameter are classified as microadenomas: those greater than 10 mm as macroadenomas. Other investigators have classified adenomas greater or equal to 40 mm in diameter, or ascending to within 5 mm of the foramen of Monroe as giant adenomas (7). Although the original classification was based on lateral skull tomograms, these have been supplanted by computed tomography (CT) and magnetic resonance imaging (MRI) without formally altering the classification scheme.
| Table 2. Classification of pituitary adenomas | |||
| Radiologic | Anatomic | Surgical | |
| Sella Turcica | |||
| Grade 0 | Intact, normal contour | Micro | Enclosed |
| Grade I | Intact, focal bulging | ||
| Grade II | Intact, enlarged | ||
| Grade III | Destroyed, partially | ||
| Grade IV | Destroyed, totally | ||
| (Grade V) | (Distant spread through cerebrospinal fluid or blood |
||
| Extrasellar Extension | |||
| Suprasellar (symmetric) |
|||
| A. Suprasellar cistern B. Recesses third ventricle C. Whole anterior third ventrical |
|||
| Parasellar (asymetric) | |||
| D. Intracranial-intradural Anterior Midline Posterior E. Extracranial-Extradural (Lateral cavernous) |
|||
Microadenomas are classified as being grade 0 or I depending on the presence of sellar bulging. Macroadenomas are graded from II to IV based on the degree of sellar enlargement and destruction. Macroadenomas are further staged A to E according to the degree of suprasellar and extrasellar extension. This classification scheme has proven useful in surgical planning and correlates with operative risk and outcome (8).
adenomas can be classified clinically in accordance with symptomatic endocrinologic activity. Endocrinologically active adenomas are those that produce and release endocrinologically active anterior pituitary hormones such as prolactin, growth hormone, ACTH, and TSH. These typically present with symptoms of endocrinopathy. In contrast, endocrinologically inactive adenomas either fail to produce hormone (i.e., null cell adenomas); produce hormone that is inadequately secreted (i.e., a-subunit only [9]) or biologically inactive (i.e., silent corticotropic adenomas); or fail to produce symptoms (i.e., follicle-stimulating hormone or luteinizing hormone in male patients).
Pathologic grading schemes for pituitary adenomas originally used cytoplasmic staining affinities to distinguish tumor subtypes. More recent studies have demonstrated, however, that cytoplasmic staining affinities correlate not only with the contents of the secretory granules, but with their density. The classification scheme currently in use uses immunohistochemistry, supported in some cases by ultrastructural morphology, to classify adenomas into 10 types based on the storage and secretion of granules filled with pituitary hormones (10) (Table 3).
| Table 3. Pathologic classification of pituitary adenomas |
| Prolactin cell adenomas |
| Growth hormone cell adenomas |
| Mixed prolactin cell-growth hormone cell adenomas |
| Acidophilic stem cell adenomas |
| Corticotropic cell adenomas |
| Gonadotropic cell adenomas |
| Thyrotropic cell adenomas |
| Plurihormonal adenomas |
| Null cell adenomas |
The invasiveness of adenomas has been classified radiographically, surgically, and histologically. Although radiologic and gross surgical invasiveness appear to correlate with poor outcome, there appears to be no correlation between microscopic dural invasion, ploidy, mitotic index, and the aggressiveness of these neoplasms, which occasionally invade locally into bone, vessels, dura, or the brain (11).
Carcinoma of the pituitary is extremely rare, and impossible to diagnose based on histologic features alone. It is thought to arise from pituitary adenomas of all types and is defined as Hardy grade V by distant spread through blood or CSF. These tumors typically present with multiple local recurrences followed by metastatic dissemination to the subarachnoid space or extraneuronal sites such as bone, liver, lymph node, lung, or kidney.
Primary Neoplasms of the Posterior and Intermediate Lobes of the Pituitary
The neurohypophysis is rarely the site of clinically significant primary neoplasms. The most common of these is the granular cell tumor, also referred to as choristoma. The histogenesis of these lesions is uncertain, but they are found in 6.8% to 17% of pituitary glands of asymptomatic people at autopsy (12), and may also occur in the stalk. They most often present in the fifth decade of life. Gliomas, gangliocytomas, and hamartomas of the neurohypophysis are extremely rare (13).
Craniopharyngiomas
Craniopharyngiomas comprise 1% to 3% of intracranial tumors. They most commonly present as suprasellar masses, although approximately 20% are primarily intrasellar, and they are the most frequent parasellar lesions of children and adolescents and the second most common sellar lesions in adults. They are thought to be derived from cell rests of the intermediate lobe that arise from remnants of Rathkes pouch and are typically filled with a viscous fluid, rich in lipid and cholesterol, grossly resembling motor oil. They are frequently calcified and surrounded by intense gliotic reaction.
Meningiomas
Meningiomas are the third most common sellar lesions, comprising 1% to 3% of lesions in this region (14). Peak incidence of meningiomas is between 40 and 50 years of age, and women outnumber men by fourfold or more. Purely intrasellar meningiomas are rare (15). More commonly, meningiomas arising at the tuberculum sellae, medical sphenoid wing, olfactory groove, diaphragma sellae, or cavernous sinus invade the sellar and parasellar structures.
Chordomas
Chordomas are slowly growing, expansile, extradural neoplasms thought to arise from notochordal remnants. Median age at presentation is 45 years, and there is a slight male predominance. About 40% arise in the skull base, and of these, 33% involve the sellar or parasellar region (16). These tumors typically originate in the clivus and erode through the posterior clinoids and dorsum sellae into the sella and sphenoid sinus. Primary sellar lesions also occur (16, 17). Metastatic dissemination is a late occurrence in 10% to 20% of cases.
Other Primary Neoplasms
Gliomas of the optic nerve and chiasm, germ cell tumors, and hamartomas also occur in the parasellar region and should be considered in the differential diagnosis of sellar lesions, particularly in children. Epidermoid and dermoid cysts comprise approximately 1% and 0.1%, respectively, of all intracranial neoplasms, and occasionally present as sellar lesions in adults.
Metastatic Neoplasms of the Sella
The sella is a common site of systemic metastasis. Gross evidence of sellar metastasis occurs in 1% to 10% of patients dying of systemic cancer (10), although microscopic analysis reveals an incidence approaching 27% (18, 19). However, sellar metastases usually occur in the context of advanced systemic disease and are rarely symptomatic (20). The most common manifestation of metastatic disease is osseous involvement of the sella. Metastatic deposits also occur in the pituitary gland and neurohypophysis, but two thirds of these result from contiguous spread. Hematopoietic metastases commonly arise as meningeal lesions, particularly in non-Hodgkins lymphoma (21).
Nonneoplastic Lesions
Cysts
Benign cysts of the pituitary are identified in 13% to 23% of routine autopsies (22). These include Rathkes cleft cysts, mucoceles, and arachnoid cleft cysts.
Rathkes cleft cysts are epithelial cysts derived from the remnants of Rahtkes pouch. Although the involution of the craniopharyngeal duct is usually accompanied by the involution of Rathkes pouch, persistent, discontinuous, cystic remnants of the intermediate lobe are common. These usually are not large enough to be symptomatic. Those that are, presumably grow by progressive accumulation of their colloidal content. They are typically lined with cuboidal epithelium, but cysts lined with squamous epithelium indistinguishable from craniopharyngioma have also been described. They present over a wide range of ages and have no gender predilection.
Mucoceles are epithelial cysts arising from the paranasal sinuses. They are histologically indistinguishable from Rathkes cleft cysts, and differentiation relies on anatomic evidence of extension from a paranasal sinus obtained radiologically or at surgery.
Arachnoid cysts consist of distended areas of arachnoid filled with CSF that occasionally become symptomatic because of mass effect. They can be sellar, suprasellar, or both. Their pathogenesis is unclear: some believe them to be congenital, whereas others contend they are acquired.
Inflammatory and Infectious Lesions
Inflammatory Lesions
The Most common inflammatory lesions of the pituitary are lymphocytic hypophysitis, Langerhans cell histiocytosis, giant cell granuloma, and sarcoidosis. Lymphocytic hypophysitis is a destructive inflammatory process affecting the anterior pituitary that is presumed to be an autoimmune phenomenon. It occurs mainly in women, either during pregnancy or in the first postpartum year, and is often associated with autoimmune diseases of other endocrine glands. It also occurs in older patients of both genders (23).
Langerhans cell histiocytosis, formerly known as histiocytosis X, consists of a group of poorly understood eosinophilic granulomatous disorders that may be localized or diffuse. Involvement of the central nervous system in patients with disseminated disease is not uncommon, although isolated lesions of the sella are extremely rare (13, 24). These granulomas appear to have a predilection for the stalk and adenohypophysis; the posterior pituitary is usually spared.
Giant cell granuloma and sarcoidosis are also granulomatous lesions. Giant cell granulomas are noncaseating granulomas of the adenohyp-ophysis and infundibular stalk. They are typically multiple and have neither a sexual predilection nor an association with pregnancy (25). Sarcoidosis of the central nervous system occurs in 5% of patients with systemic sarcoidosis. Although neuritis is the most common presentation, inflammation of both the anterior and posterior lobes of the pituitary, as well as the stalk and hypothalamus, also occurs in about 1% of patients (26).
Infectious Lesions
Infection of sellar structures occurs only rarely. Bacterial abscesses may arise hematogenously or by secondary extension from an anatomically contiguous focus. Acute sphenoid inusitis or osteomyelitis are the most likely niduses for contiguous spread. The most frequent organisms are Staphylococcus aureus, Streptococcus pneumoniae, group A Streptococcus, and Klebsiella (27). There seems to be a predilection or abscesses to occur in the bed of a preexisting sellar lesion such as pituitary adenoma or craniopharyngioma (28). Tuberculosis, still endemic to certain areas, typically involves the sella in a dense, basilar meningitis, with or without arthritis. Intrasellar tuberculomas are usually associated with systemic tuberculosis and can result in pituitary destruction. spergillosis may also present with an inflammatory sellar mass (29), as can parasitic diseases such as cysticercosis (30) or echinococcosis (31).
Vascular Lesions
Aneurysms of the sellar region usually derive from the infraclinoid or intracavernous carotid artery. They are readily diagnosed by MRI and mentioned here merely as a diagnostic consideration to be excluded.
Clinical Presentation
Patients with sellar lesions present with a myriad of symptoms and signs. These can be classified as general or specific.
General Symptoms and Signs
The general symptoms and signs produced by sellar lesions result from mass effect, which stretches or compresses the densely packed neurovascular structures of the sella. These structures include the internal carotid artery and its proximal branches; the optic nerve, chiasm, and tracts; the pituitary gland; the infundibular stalk; the hypothalamus; the cavernous sinus and the cranial nerves within; the frontal and medial temporal lobes; the pons and midbrain; and the ventricular system.
Visual Manifestations
The most common manifestation of sellar or parasellar lesions in adults is visual impairment. Chiasmal compression produces a deficit of the bilateral superior temporal visual quadrant initially, followed by involvement of the inferior temporal quadrant. A junctional scotoma consisting of ipsilateral central scotoma with a contralateral superior temporal quadrantanopsia may also be produced by involvement of optic nerve fibers crossing into the chiasm at acute angles (Von Willebrands knee). Further compression may lead to irreversible chiasmal damage, resulting in total blindness. Isolated compression of the prechiasmal optic nerve results in an afferent pupillary defect known as Marcus Gunns sign.
Hypothalamic and Pituitary Dysfunction
Compression of the hypothalamus may lead to dysregulation of pituitary secretion as well as a myriad of other physiologic processes regulated by the hypothalamus. These include water balance, body temperature, level of consciousness, and behavior.
Compression of the median eminence or infundibular stalk may result in the impairment of synthesis of hypophysiotropic hormones or their transport to the pituitary gland itself. This results in varying degrees of hypopituitarism as well as diabetes insipidus. By interfering with the transport of dopamine the predominant prolactin-inhibiting factor to the lactotrophs, stalk compression also produces a paradoxical mild to moderate hyperprolactinemia (typically 30 150 ng/ml). This may present as hypogonadism or galactorrhea in women or men.
Compression of the pituitary gland itself also commonly results in hypopituitarism. The clinical symptoms depend on the cell types affected, and the degree of hyposecretion. The gonadotrophs are the most vulnerable and are usually affected first, leading to amenorrhea or oligomenorrhea in women, gonadal atrophy in men, and loss of libido in both sexes. Thyrotrophs and somatotrophs are usually affected next. Corticotrophs have the greatest functional reserve, and addisonian symptoms of nausea, vomiting, and postural hypotension usually present only in severe panhypopituitarism.
Cavernous Sinus Involvement
Sellar lesions extending into the cavernous sinus may involve the third, fourth, and sixth cranial nerves, resulting in partial or complete ophthalmoplegia. This is more commonly found in meningiomas than pituitary adenomas or craniopharyngiomas. The oculomotor nerve is the most commonly affected nerve in the cavernous sinus, followed by the abducens and trochlear nerves. Involvement of the first two branches of the trigeminal nerve results in numbness or pain in these distributions.
Headache
Headache results from stretching or compressing the dura of the diaphragma sellae, tentorium, cavernous sinus, or the base of the skull, all of which are innervated by the first division of the trigeminal nerve. Other mechanisms by which sellar lesions produce headaches are inflammation or pressure on other pain-sensitive dural structures, and elevated intracranial pressure due to mass effect or obstructive hydrocephalus.
Hydrocephalus
Sellar lesions with significant suprasellar extension may obstruct the foramen of Monro, producing unilateral or bilateral obstructive hydrocephalus. This may result in headache, papilledema, and decreased levels of consciousness. Stretching of the sixth cranial nerve may also produce an abducens palsy.
Cortical Involvement
Sellar lesions with significant suprasellar or parasellar extension may compress adjacent brain. Usually, slow-growing lesions reach large size before becoming symptomatic. Compression of the frontal lobes produces personality change and memory loss, whereas temporal compression or irritation may induce seizure.
Apoplexy
Pituitary apoplexy is a clinical manifestation of hemorrhage or infarction of a pituitary adenoma. The syndrome is characterized by the acute onset of severe headache and meningismus, often accompanied by nausea and vomiting. Partial or complete ophthalmoplegia is common, and a decreased level of consciousness may also occur. This clinical emergency results from the acute onset of mass effect on parasellar structures such as the optic chiasm or the cavernous and appears to be more frequent in clinically non-functional macroadenomas.
Specific Symptoms and Signs
Specific symptoms and signs may be diagnostic of certain endocrinologically active pituitary adenomas.
Prolactinomas
Prolactinomas account for about 30% to 40% of all pituitary adenomas and are the most commonly encountered endocrinologically active adenomas. They present differently in men and women. Women present threefold more frequently than men and complain of oligomenorrhea or amenorrhea, often with infertility and galactorrhea. They are typically 20 to 30 years of age and have microadenomas with moderately elevated prolactin levels (>200 ng/ml). In contrast, men typically present between 40 and 50 years of age, with general symptoms of mass effect. Men often harbor invasive mactoadenomas with significantly higher prolactin levels. They often admit to decreased libido or impotence, but rarely present with this as a chief complaint. Galactorrhea also occurs occasionally in men.
Growth Hormone-Secreting Adenomas
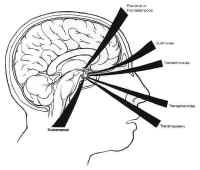 Figure 9: Surgical approaches to the sella turcicaExcess growth hormone due to pituitary adenoma results in giganticism if manifested in childhood before fusion of the epiphysis of the long bones. In adults, these tumors present with acromegaly, resulting in gross enlargement of soft tissues of the face and the extremities. Bone density also increases, resulting in degenerative joint disease, spinal stenosis, and compressive neuropathies. Acromegaly is also associated with systemic illness, including brittle diabetes, hypertension, severe atherosclerosis, and organomegaly. Poorly understood respiratory disease and cardiomyopathy result in decreased life expectancy for these patients.
Figure 9: Surgical approaches to the sella turcicaExcess growth hormone due to pituitary adenoma results in giganticism if manifested in childhood before fusion of the epiphysis of the long bones. In adults, these tumors present with acromegaly, resulting in gross enlargement of soft tissues of the face and the extremities. Bone density also increases, resulting in degenerative joint disease, spinal stenosis, and compressive neuropathies. Acromegaly is also associated with systemic illness, including brittle diabetes, hypertension, severe atherosclerosis, and organomegaly. Poorly understood respiratory disease and cardiomyopathy result in decreased life expectancy for these patients.
Adrenocorticotropic Hormone-Secreting Adenomas
Hypercortisolism, or Cushings syndrome, results in a myriad of clinical features, including moon facies, centripetal justments of head to facilitate improved visualization. Further flexion of the head improves access to the posterior sella and clivus, whereas extension improves exposure of the tuberculum and planum sphenoidale. The left thigh is positioned on a bolster to expose the superolateral aspect of the leg from the hip to the knee to facilitate a graft of fat and fascia lata, if needed. In very thin people, it may rarely be necessary to remove additional fat from the anterior abdominal wall. A lumbar subarachnoid drain may also be inserted before surgery for injection of saline or air to aid in bringing down a suprasellar mass. This has been used extensively by some surgeons (43) but is rarely done at the authors institution. Preoperative ventriculostomy may also be indicated for patients with hydrocephalus due to obstruction of the foramen of Monro or the third ventricle by a suprasellar mass. The fluoroscopy unit may also be used, although this is usually required only for patients with a conchal sphenoid sinus or patients lacking the usual anatomic landmarks, such as patients who have previously undergone transsphenoidal surgery or rhinoplasty.
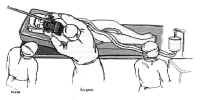 Figure 10: Position of the patient and operating team for the transnasal-transsphenoidal approachThe face and mouth are prepared with povidone-iodine from the bridge of the nose to the jaw to create a “semi-sterile” field. A vaginal pack is inserted in the oropharynx to prevent blood from entering the throat, and the gingiva and nasal mucosa are injected with 0.5% lidocaine with 1/200,000 epinephrine using a 25-gauge spinal needle until they blanch. The sphenopalatine ganglia and the ethmoid nerves are blocked with 1 x 3 cm cottonoids soaked with 4% cocaine to vasoconstrict the mucosa and shrink the nasal turbinates. Usually, the dissection is done on the left side for the convenience of the right-handed surgeon. However, if the septum is deviated significantly to the left, the right side should be used. Because the thigh is a sterile field, it is prepared separately. Two separate operative fields are maintained each with a separate set of instruments that are not to be shared.
Figure 10: Position of the patient and operating team for the transnasal-transsphenoidal approachThe face and mouth are prepared with povidone-iodine from the bridge of the nose to the jaw to create a “semi-sterile” field. A vaginal pack is inserted in the oropharynx to prevent blood from entering the throat, and the gingiva and nasal mucosa are injected with 0.5% lidocaine with 1/200,000 epinephrine using a 25-gauge spinal needle until they blanch. The sphenopalatine ganglia and the ethmoid nerves are blocked with 1 x 3 cm cottonoids soaked with 4% cocaine to vasoconstrict the mucosa and shrink the nasal turbinates. Usually, the dissection is done on the left side for the convenience of the right-handed surgeon. However, if the septum is deviated significantly to the left, the right side should be used. Because the thigh is a sterile field, it is prepared separately. Two separate operative fields are maintained each with a separate set of instruments that are not to be shared.
There are two variations of the transsphenoidal approach, the transseptal and the sublabial. The transseptal incision is quicker but gives a narrower exposure. It is useful when the nostril is large and the tumor is small. The sublabial incision, which the authors use exclusively, was introduced by Cushing and gives a wider exposure with better access to the superior aspect of the sella. Using a headlight for illumination, the gingival mucosa is incised from canine to canine down to the periosteum the maxilla (Fig. 11A). The mucoperiosteum is then dissected superiorly in a subperiosteal fashion to expose the pyriform aperture. The spine and any superomedial extension of the maxillary rostrum is then removed with a Kerrison rongeur as required to flatten the view of the floor of the nose (see Fig 11B).
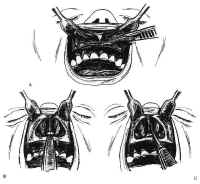 Figure 11: Transsphenoidal approach to the sella turcica. A: The gingival mucosa is incised from canine to canine with no. 15 blade. B: After dissection, the pyriform aperture is enlarged as required. C,D: The mucosa is elevated off the left side of the quadrangular cartilage ventral to the anterior boarder of the perpendicular plate. E: After freeing the quadrangular cartilage from the vomer and the perpendicular plate, the left anterior and inferior submucosal tunnels are sharply dissected, and the quadrangular cartilage is retracted to the right using the nasal speculum.Bilateral inferior tunnels are made first. The mucosa is first dissected from the nasal floor up to the maxillary crest of the septum using Cottle, Woodson, and Freer elevators. Next, the quadrangular cartilage is exposed sharply in the midline by incising the mucosa with a no. 15 blade. The subperiosteal dissection then proceeds posteriorly and superiorly to free the quadrangular cartilage from the interdigitation of the septal perichondrium with the periosteum of the maxillary crest (see Fig. 11C). These connections are typically concentrated along the junctions between the crest, the vomer, and the perpendicular plate of the ethmoid. The mucoperichondrium of the septal cartilage is extended beyond its bony septal attachments. This creates an anterior submucosal tunnel. The connective tissue bands connecting the quadrangular cartilage to the superior vomer and the anterior perpendicular plates are then sharply dissected with a Freer knife (see Fig. 11D). The quadrangular cartilage is then retracted laterally to the right with the nasal speculum (see Fig. 11E). The contralateral nasal mucosa and superior aspect of the quadrangular cartilage remain intact. The mucosa of the nasal floor is elevated into the inferior meatus on each side. The perpendicular plate is fractured, removed, and saved for possible use during closure. As the final septal mucoperiosteal dissection proceeds over the vomer to the posterior termination of the nasal septum, nasal specula of increasing length are used for the exposure. Care is taken to provide wide access to the sphenoid rostrum superiorly. By preserving a small, short spike of vomer or ethmoid plate protruding from the maxillary crest, a guidepost is maintained to the midline of the nasal cavity.
Figure 11: Transsphenoidal approach to the sella turcica. A: The gingival mucosa is incised from canine to canine with no. 15 blade. B: After dissection, the pyriform aperture is enlarged as required. C,D: The mucosa is elevated off the left side of the quadrangular cartilage ventral to the anterior boarder of the perpendicular plate. E: After freeing the quadrangular cartilage from the vomer and the perpendicular plate, the left anterior and inferior submucosal tunnels are sharply dissected, and the quadrangular cartilage is retracted to the right using the nasal speculum.Bilateral inferior tunnels are made first. The mucosa is first dissected from the nasal floor up to the maxillary crest of the septum using Cottle, Woodson, and Freer elevators. Next, the quadrangular cartilage is exposed sharply in the midline by incising the mucosa with a no. 15 blade. The subperiosteal dissection then proceeds posteriorly and superiorly to free the quadrangular cartilage from the interdigitation of the septal perichondrium with the periosteum of the maxillary crest (see Fig. 11C). These connections are typically concentrated along the junctions between the crest, the vomer, and the perpendicular plate of the ethmoid. The mucoperichondrium of the septal cartilage is extended beyond its bony septal attachments. This creates an anterior submucosal tunnel. The connective tissue bands connecting the quadrangular cartilage to the superior vomer and the anterior perpendicular plates are then sharply dissected with a Freer knife (see Fig. 11D). The quadrangular cartilage is then retracted laterally to the right with the nasal speculum (see Fig. 11E). The contralateral nasal mucosa and superior aspect of the quadrangular cartilage remain intact. The mucosa of the nasal floor is elevated into the inferior meatus on each side. The perpendicular plate is fractured, removed, and saved for possible use during closure. As the final septal mucoperiosteal dissection proceeds over the vomer to the posterior termination of the nasal septum, nasal specula of increasing length are used for the exposure. Care is taken to provide wide access to the sphenoid rostrum superiorly. By preserving a small, short spike of vomer or ethmoid plate protruding from the maxillary crest, a guidepost is maintained to the midline of the nasal cavity.
The long Cottle sepculum is now replaced by the Ha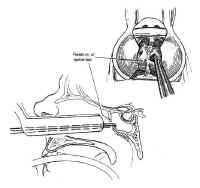 Figure 12: The sphenoid ostia are identified and enlarged using a rongeur. The entire rostrum of the sphenoid is removed in this fashion.rdy retractor. Careful dissection of mucoperiosteum over the face of the sphenoid is carried down to the sphenoid sinus ostia exposes bilaterally. The ostia are opened with a Kerrison rongeur until the sphenoid face begins to change its attitude from a vertical to a horizontal plane (Fig. 12). The speculum is continuously readjusted with the help of the “persuader” to capture the sphenoid face mucosa precisely under the tips of the retractor and to prevent “ballooning” of the mucosa into the visual field. Placement of the speculum in the sphenoid sinus should be avoided because fractures may result in traction damage to the optic nerve or the carotid artery. The entire anterior wall of the sphenoid sinus is removed. In patients with a conchal sphenoid sinus, a high-speed drill is used to open the sphenoid bone using fluoroscopic monitoring. Fluoroscopy may also be useful in other patients whose bony anatomy has been similarly obscured by previous transsphenoidal surgery or rhinoplasty. The sphenoid sinus mucosa is then removed using a pituitary rongeur or Decker.
Figure 12: The sphenoid ostia are identified and enlarged using a rongeur. The entire rostrum of the sphenoid is removed in this fashion.rdy retractor. Careful dissection of mucoperiosteum over the face of the sphenoid is carried down to the sphenoid sinus ostia exposes bilaterally. The ostia are opened with a Kerrison rongeur until the sphenoid face begins to change its attitude from a vertical to a horizontal plane (Fig. 12). The speculum is continuously readjusted with the help of the “persuader” to capture the sphenoid face mucosa precisely under the tips of the retractor and to prevent “ballooning” of the mucosa into the visual field. Placement of the speculum in the sphenoid sinus should be avoided because fractures may result in traction damage to the optic nerve or the carotid artery. The entire anterior wall of the sphenoid sinus is removed. In patients with a conchal sphenoid sinus, a high-speed drill is used to open the sphenoid bone using fluoroscopic monitoring. Fluoroscopy may also be useful in other patients whose bony anatomy has been similarly obscured by previous transsphenoidal surgery or rhinoplasty. The sphenoid sinus mucosa is then removed using a pituitary rongeur or Decker.
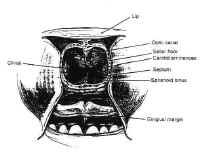 Figure 13The bulge of the sella is readily appreciated, as is the floor of the optic canal and the bony prominence of the carotid arteries (Fig. 13). Often, expansile sellar masses erode through the sellar floor, and resection can begin after merely opening the anterior wall of the sphenoid sinus. The carotid arteries may erode through the bone as well, however, particularly in older, hypertensive patients. When the sellar wall is intact, it is perforated and removed using a small osteotome and Kerrison rongeurs. The sphenoid septa are also removed when present. The bony resection can be extended superiorly to the tuberculum when there is a large degree of suprasellar extension.
Figure 13The bulge of the sella is readily appreciated, as is the floor of the optic canal and the bony prominence of the carotid arteries (Fig. 13). Often, expansile sellar masses erode through the sellar floor, and resection can begin after merely opening the anterior wall of the sphenoid sinus. The carotid arteries may erode through the bone as well, however, particularly in older, hypertensive patients. When the sellar wall is intact, it is perforated and removed using a small osteotome and Kerrison rongeurs. The sphenoid septa are also removed when present. The bony resection can be extended superiorly to the tuberculum when there is a large degree of suprasellar extension.
After the bony resection is complete, the dura is coagulated transversely with a bipolar cautery, then incised in a cruciate manner (Fig. 14). The intercavernous sinuses are often encountered at the superior and inferior margins of exposure, resulting in brisk bleeding. This is controlled by coagulating the leaves of the dura with a bipolar cautery.
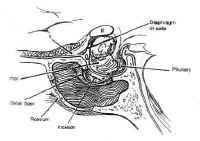 Figure 14: After removing the sellar floor with rongeurs, the dura is opened in a cruciate fashion.Once access to the sella has been achieved, the lesion can be sampled for biopsy and gently resected using Hardy microinstruments, bayonetted ring curettes, and aspiration. Pituitary adenomas are non-encapsulated, soft, and gelatinous, with the consistency of jam, and are readily removed using this technique. Great care is taken to preserve the normal gland, which is often seen as orange-tinged tissue abutting the stalk or the posterior wall of the sella. Cystic lesions, such as craniopharyngiomas, are drained with a needle under direct visualization before resecting the solid portion of the mass. Intermittent Valsalva maneuver by the anesthesiologist or injection of saline or air through a lumbar drain expedites tumor removal by elevating the intracranial pressure, which forces the mass inferiorly. The integrity of the arachnoid is maintained, when possible, to avoid a CSF fistula.
Figure 14: After removing the sellar floor with rongeurs, the dura is opened in a cruciate fashion.Once access to the sella has been achieved, the lesion can be sampled for biopsy and gently resected using Hardy microinstruments, bayonetted ring curettes, and aspiration. Pituitary adenomas are non-encapsulated, soft, and gelatinous, with the consistency of jam, and are readily removed using this technique. Great care is taken to preserve the normal gland, which is often seen as orange-tinged tissue abutting the stalk or the posterior wall of the sella. Cystic lesions, such as craniopharyngiomas, are drained with a needle under direct visualization before resecting the solid portion of the mass. Intermittent Valsalva maneuver by the anesthesiologist or injection of saline or air through a lumbar drain expedites tumor removal by elevating the intracranial pressure, which forces the mass inferiorly. The integrity of the arachnoid is maintained, when possible, to avoid a CSF fistula.
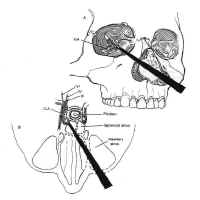 Figure 15: The extended transsphenoidal approach for lesions extending lateral to the carotid artery (Hardy grade D). The pyriform aperture is enlarged contralateral to the lesion, facilitating improved lateral exposure of the neoplasm.When lesions involve only the sella or the medial cavernous sinus, the standard transsphenoidal approach is sufficient. However, when lesions extend more laterally into the cavernous sinus (Hardy grade D), exposure can be improved by enlarging the pyriform aperture or performing a medial maxillotomy contralateral to the invaded cavernous sinus (44). This allows the speculum and the resection itself to be directed laterally (Fig. 15). The medial wall of the cavernous sinus consists of a single thick layer, contiguous with the lateral layer of sellar dura. Neoplasms invading the medial cavernous sinus typically obliterate the venous sinuses and can be removed using fine ring curettes and microdissectors. These instruments can be passed around the carotid artery to remove tumor that has extended laterally. Significant venous bleeding is usually not encountered until the patent portion of the cavernous sinus is exposed in the final phase of the resection, and is well controlled with Surgicel and direct pressure. Although this technique can often be used safely to resect neoplasm lateral to the carotid, narrowing of the caliber of the cavernous carotid on MRI suggests invasion of the vessel wall and is a relative contraindication to aggressive exploration using this technique.
Figure 15: The extended transsphenoidal approach for lesions extending lateral to the carotid artery (Hardy grade D). The pyriform aperture is enlarged contralateral to the lesion, facilitating improved lateral exposure of the neoplasm.When lesions involve only the sella or the medial cavernous sinus, the standard transsphenoidal approach is sufficient. However, when lesions extend more laterally into the cavernous sinus (Hardy grade D), exposure can be improved by enlarging the pyriform aperture or performing a medial maxillotomy contralateral to the invaded cavernous sinus (44). This allows the speculum and the resection itself to be directed laterally (Fig. 15). The medial wall of the cavernous sinus consists of a single thick layer, contiguous with the lateral layer of sellar dura. Neoplasms invading the medial cavernous sinus typically obliterate the venous sinuses and can be removed using fine ring curettes and microdissectors. These instruments can be passed around the carotid artery to remove tumor that has extended laterally. Significant venous bleeding is usually not encountered until the patent portion of the cavernous sinus is exposed in the final phase of the resection, and is well controlled with Surgicel and direct pressure. Although this technique can often be used safely to resect neoplasm lateral to the carotid, narrowing of the caliber of the cavernous carotid on MRI suggests invasion of the vessel wall and is a relative contraindication to aggressive exploration using this technique.
After maximal res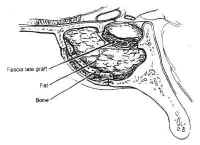 Figure 16: Repair of cerebrospinal fluid (CSF) fistula. If a CSF leak is identified during surgery, it may be patched with fascia lata and held in place with Gelfoam or fat, tamponaded with bone.ection has been completed, the sella and the pituitary gland itself are systematically inspected. If the tumor capsule prolapses into the sphenoid sinus, or there is a gross CSF leak visible, fascia lata, fat, and bone are used to reconstruct the sellar floor (Fig. 16). The fascia lata is placed in the sellar opening and sealed with fibrin glue after packing the sella with a small amount of fat or Surgicel. The fascia is then buttressed within the sella by the wedge of bony septum removed earlier. The integrity of the closure is then tested by having the anesthesiologist maintain a Valsalva maneuver at 40 mm Hg pressure. If the closure is satisfactory, the speculum is removed and the nasal mucosa is reapproximated and tamponaded using gauze impregnated with petroleum jelly. The gingival mucosa is reapproximated using 3-0 chromic suture, and a “mustache” dressing consisting of a folded gauze is placed below the nostrils. In cases with extensive CSF leaks, lumbar drainage is continued for 2 to 3 days. For large suprasellar lesions, the sella is packed regardless of whether a CSF leak is present. This is necessary because the resection may produce an “empty sella” secondarily. This may result in collapse of the diaphragma sellae and consequent traction on the chiasm, leading to decreased vision.
Figure 16: Repair of cerebrospinal fluid (CSF) fistula. If a CSF leak is identified during surgery, it may be patched with fascia lata and held in place with Gelfoam or fat, tamponaded with bone.ection has been completed, the sella and the pituitary gland itself are systematically inspected. If the tumor capsule prolapses into the sphenoid sinus, or there is a gross CSF leak visible, fascia lata, fat, and bone are used to reconstruct the sellar floor (Fig. 16). The fascia lata is placed in the sellar opening and sealed with fibrin glue after packing the sella with a small amount of fat or Surgicel. The fascia is then buttressed within the sella by the wedge of bony septum removed earlier. The integrity of the closure is then tested by having the anesthesiologist maintain a Valsalva maneuver at 40 mm Hg pressure. If the closure is satisfactory, the speculum is removed and the nasal mucosa is reapproximated and tamponaded using gauze impregnated with petroleum jelly. The gingival mucosa is reapproximated using 3-0 chromic suture, and a “mustache” dressing consisting of a folded gauze is placed below the nostrils. In cases with extensive CSF leaks, lumbar drainage is continued for 2 to 3 days. For large suprasellar lesions, the sella is packed regardless of whether a CSF leak is present. This is necessary because the resection may produce an “empty sella” secondarily. This may result in collapse of the diaphragma sellae and consequent traction on the chiasm, leading to decreased vision.
After surgery, patients are managed in the neurosurgery step-down unit, where close attention is paid to the ophthalmologic examination and the detection of diabetes insipidus. New deficits are rare, and improvement in the visual field examination is sometimes noted early in the postoperative period. Diabetes insipidus usually does not appear before 12 to 24 hours after surgery, and polyuria is not treated unless the diagnosis of diabetes insipidus is clear: urine output greater than 300 ml/hour for 2 consecutive hours with a specific gravity less than 1.005, urine osmolarity of 50 to 150 mOsm/L, and a serum Na of at least 145 and rising. The patient is allowed to drink ad libitum and treated with aqueous DDAVP (vasopressin), 1 ug every 12 to 24 hours intravenously or subcutaneously as needed. In the immediate postoperative period, serum sodium and osmolarity and urine output are monitored closely. The patients are encouraged to ambulate the night of surgery, and the Foley catheter is removed after 24 hours unless diabetes insipidus makes this inconvenient. Intravenous antibiotics are continued until the nasal packing is removed on the third postoperative day, after which the patient undergoes a postoperative MRI. Patients without complications are routinely sent home on the second or third postoperative day on a tapering dose of steroids, with follow-up appointments for both the neurosurgery and endocrinology clinics.
The most frequent complications of the transsphenoidal approach are CSF leaks and damage to the carotid artery or cranial nerves. CSF leaks can usually be avoided using the techniques outlined previously. Persistent leaks are usually controlled with prolonged lumbar drainage and elevation of the head. Re-operation for repair of a CSF leak refractory to conservative management is sometimes required and is usually done using the TNTS approach. Tension pneumocephalus, a related complication, occurs because of continual accumulation of intracranial air through a dural tear with a ball-value mechanism that prevents its egress. This is far more rare, but more deadly, and this possibility should be considered inpatients whose postoperative mental status deteriorates, particularly when a lumbar drain is in place. After the lumbar drainage is discontinued, and the diagnosis is confirmed, the patient is placed on 100% oxygen. The pneumocephalus gradually resolves over several days. Tension pneumocephalus may also be decompressed with a spinal needle transnasally or transcranially in emergent circumstances.
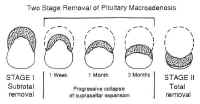 Figure 17: Two-stage removal of pituitary macroadenoma with suprasellar extension.Laceration of the intracavernous carotid is another rare but serious complication that occurs more frequently in cases requiring cavernous sinus exploration. Because of the narrowness of the field, temporary occlusion and primary repair of the carotid are impossible in the transsphenoidal approach. Lacerations are treated by holding direct pressure with Surgicel and cotton pledgets until the bleeding stops. An intra operative angiogram should be obtained when possible to document patency of the carotid and rule out contrast extravasation. Follow-up angiograms should be obtained before discharge and at 3 months to rule out pseudoaneurysms. Cranial nerve palsies as well as lesions of the brainstem and hypothalamus are also possible, although rare. After ruling out hematoma or pneumocephalus, they are managed expectantly. Recovery is variable.
Figure 17: Two-stage removal of pituitary macroadenoma with suprasellar extension.Laceration of the intracavernous carotid is another rare but serious complication that occurs more frequently in cases requiring cavernous sinus exploration. Because of the narrowness of the field, temporary occlusion and primary repair of the carotid are impossible in the transsphenoidal approach. Lacerations are treated by holding direct pressure with Surgicel and cotton pledgets until the bleeding stops. An intra operative angiogram should be obtained when possible to document patency of the carotid and rule out contrast extravasation. Follow-up angiograms should be obtained before discharge and at 3 months to rule out pseudoaneurysms. Cranial nerve palsies as well as lesions of the brainstem and hypothalamus are also possible, although rare. After ruling out hematoma or pneumocephalus, they are managed expectantly. Recovery is variable.
Because of the versatility and low morbidity of the TNTS approach, it can be used even in the presence of extensive suprasellar extension. When the lesion is soft (as pituitary adenomas usually are), the suprasellar mass often collapses into the sella, where it can be safely resected at the initial procedure. Occasionally, when the tumor does not descend immediately, the residual neoplasm may collapse further into the sella with time (Fig. 17), allowing the residual to be resected by repeat TNTS if further surgery is indicated.
Facial Degloving and Extended Maxillotomy Approaches
Lesions invading the superior clivus above the hard palate and medial to the carotid arteries, such as chordomas, medial chondrosarcomas, and juvenile angiofibromas can be approached using facial degloving or extended maxillotomy approaches either alone or in combination.
Transsphenoethmoidal Approach
The transsphenoethmoidal approach to the sella was introduced by Chiari (45). The advantage of this approach over the transsphenoidal approach is the shorter operative distance to the sella (46). However, it requires a facial incision, provides a narrower axial exposure, and carries a risk of injury to the optic nerve. One version combines this with medial maxillectomy for improved axial exposure of the sella (47). However, this combination holds little benefit over maxillotomy with midface degloving, which has a lower risk and superior cosmesis.
A 3-cm incision is made along the medial border of the nose, beginning from the inferior margin of the eyebrow, and the lacrimal sac is retracted laterally. The anterior ethmoid artery is sacrificed, and the lamina papyracea over the lacrimal fossa is removed, as is the underlying middle turbinate. The ethmoid mucosa is removed to identify the sphenoid ostia. The anterior and posterior walls of the sphenoid are then removed to expose the sella. The operation then proceeds as in the standard TNTS approach.
Transcranial-Intradural Approaches
The indications for transcranial-intradural approaches to the sella consist primarily of patients for whom transsphenoidal surgery is contraindicated for reasons discussed previously. Lesions invading the cavernous sinus laterally, with secondary sellar invasion; lesions that frankly violate the dura; and residual suprasellar or lateral cavernous sinus lesions that could have been only partially resected from the transsphenoidal approach are additional indications for transcranial approaches. In addition, repair of CSF leaks may also require a transcranial approach.
There are several possible transcranial approaches to the sella trucica, but the most useful are the transbasal, pterional, and subtemporal approaches (see Fig. 9). The choice of approach is determined by the location of the lesion with respect to the sella, and to some extent by the personal preference of the surgeon. These approaches are discussed briefly in the following sections specifically in terms of their utility for the resection of sellar and parasellar lesions.
Transbasal Approach
 Figure 18: The subfrontal approach to the sella turcica. The tuberculum sellae is removed with a high speed drill to facilitate exposure of a suprasellar mass.The transbasal approaches to the sella are versions of the anterior subcranial approach. A unilateral or bilateral frontal craniotomy is performed with or without orbitofrontal craniotomy. The tuberculum sellae is removed anterior to the chiasm with a high-speed drill (Fig. 18). This minimizes the effect of a prefixed chiasm, when present, and gives the surgeon an unobstructed view of the sella between the optic nerves with minimal brain retraction. In contrast to the other transcranial approaches, the pituitary stalk is easily identified and avoided, as are the carotid arteries and their branches. This approach is particularly useful for extradural lesions such as esthesioneuroblastomas and craniopharyngiomas, as well as for tuberculum sellae and olfactory groove meningiomas with secondary sellar involvement. There is a high risk of anosmia due to avulsion as the olfactory nerves with this approach, as well as a small risk of optic nerve or carotid injury. Frontal lobe swelling can usually be avoided by proper positioning, lumbar drainage, and minimizing brain retraction.
Figure 18: The subfrontal approach to the sella turcica. The tuberculum sellae is removed with a high speed drill to facilitate exposure of a suprasellar mass.The transbasal approaches to the sella are versions of the anterior subcranial approach. A unilateral or bilateral frontal craniotomy is performed with or without orbitofrontal craniotomy. The tuberculum sellae is removed anterior to the chiasm with a high-speed drill (Fig. 18). This minimizes the effect of a prefixed chiasm, when present, and gives the surgeon an unobstructed view of the sella between the optic nerves with minimal brain retraction. In contrast to the other transcranial approaches, the pituitary stalk is easily identified and avoided, as are the carotid arteries and their branches. This approach is particularly useful for extradural lesions such as esthesioneuroblastomas and craniopharyngiomas, as well as for tuberculum sellae and olfactory groove meningiomas with secondary sellar involvement. There is a high risk of anosmia due to avulsion as the olfactory nerves with this approach, as well as a small risk of optic nerve or carotid injury. Frontal lobe swelling can usually be avoided by proper positioning, lumbar drainage, and minimizing brain retraction.
Pterional Approach
The pterional approa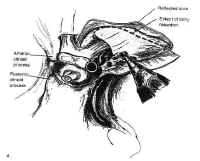 Figure 19: The pterional approach to sellar lesions. The anterior clinoid process can be removed intradurally with a high speed drill to facilitate exposure of the lateral parasellar region.ch is the shortest route to the parasellar region. This is usually done on the side of either the greatest tumor extension, or the patients worst vision. If neither of these are considerations, the craniotomy is done on the nondominant side. After performing a standard pterional craniotomy, the sphenoid ridge lateral to the superior orbital ssure is removed extradurally with a high-speed drill. The sylvian fissure is widely split and the frontal and temporal lobes are gently retracted to maximize exposure of the sellar region, and the anterior clinoid process is drilled away (Fig. 19). This gives excellent exposure of lateral parasellar lesions such as meningiomas or eccentric pituitary macroadeomas spilling into the middle fossa. Access to the sella itself, however, is somewhat impeded by the optic nerve, and carotid artery, which lie in the surgeons line of sight. In addition, the contralateral optic nerve may be poorly visualized. The risks of this approach include damage to the carotid artery as well as the optic and third cranial nerves.
Figure 19: The pterional approach to sellar lesions. The anterior clinoid process can be removed intradurally with a high speed drill to facilitate exposure of the lateral parasellar region.ch is the shortest route to the parasellar region. This is usually done on the side of either the greatest tumor extension, or the patients worst vision. If neither of these are considerations, the craniotomy is done on the nondominant side. After performing a standard pterional craniotomy, the sphenoid ridge lateral to the superior orbital ssure is removed extradurally with a high-speed drill. The sylvian fissure is widely split and the frontal and temporal lobes are gently retracted to maximize exposure of the sellar region, and the anterior clinoid process is drilled away (Fig. 19). This gives excellent exposure of lateral parasellar lesions such as meningiomas or eccentric pituitary macroadeomas spilling into the middle fossa. Access to the sella itself, however, is somewhat impeded by the optic nerve, and carotid artery, which lie in the surgeons line of sight. In addition, the contralateral optic nerve may be poorly visualized. The risks of this approach include damage to the carotid artery as well as the optic and third cranial nerves.
Subtemporal Approach
The subtemporal approach gives superior access to the posterior aspect of the sella, including the posterior clinoid process and the top of the clivus. It is most useful for lesions with retrochiasmatic extension such as chordomas and chondrosarcomas of the uppermost clivus, as well as meningiomas in that region. The approach begins with the standard pterional craniotomy. Aggressive temporal bone resection is used to minimize brain retraction. A zygomatic osteotomy may further enhance exposure. After opening the dura, the sylvian fissure is widely split, and the median temporal lobe is retracted laterally to reach the upper clivus and posterior clinoid process. The disadvantage of this approach is that the ipsilateral third and fourth cranial nerves are interposed between the surgeons line of sight and the sella. In addition, visualization of the contralateral posterior cerebral artery (PCA) and superior cerebellar artery (SCA) is poor. The major risk of this approach is temporal lobe damage due to excessive retraction.
The aforementioned three approaches can also be combined.
Adjunctive Surgical Techniques: Frameless Stereotaxy
The authors have also employed frameless stereotactic devices extensively for complex cases. This has been useful for intraoperative determination of the trajectory required for resection of certain skull base lesions, allowing the exposure to be optimized. It is also useful for intraoperative confirmation of the limits of resection and for determining the proximity of critical neurovascular structures in regions with distorted anatomy. Although brain “shift” after the dura is opened (48) limits the accuracy of this technique for deep intraparenchymal lesions, this technique is accurate for localizing skull base lesions.
TREATMENT
Pituitary Adenomas
The goals of treatment for patients with pituitary adenomas are to remove the mass effect (if present) and to normalize or restore hormonal function. Endocrinologically inactive microadenomas are usually discovered incidentally and rarely require treatment. The treatment of endocrinologically active microadenomas depends on their endocrine activity. Pharmacologic therapy had proven useful for shrinking some of these adenomas and reducing excess hormone levels. However, none of these cytotoxic agents is curative, and symptoms usually resume shortly after the drug is discontinued. Surgery, particularly through the transsphenoidal approach, is effective and relatively benign, and remains the mainstay of therapy for most pituitary adenomas other than prolactinomas. Radiation therapy has a delayed effect and is complicated by damage to the optic nerves and hypothalamic-pituitary axis as well as by radiation necrosis. Impaired mental status and radiation-induces tumors have also been described. Focused-beam radiation therapy is useful in patients with recurrent or residual tumor, or poor operative candidates whose tumors cannot be controlled with medical therapy. The treatment of specific subtypes of pituitary adenoma is addressed in the following sections.
Prolactinomas
The treatment of prolactinomas remains controversial. Medical therapy with bromocriptine, and ergot derivative, not only shrinks the tumor but also decreases prolactin synthesis and release (49), and is considered first-line therapy for most prolactinomas. This produces relief of symptoms, including galactorrhea and amenorrhea, and can restore fertility. Although accelerated tumor growth can occur during pregnancy, this is rarely significant in microprolactinomas. There have been no teratogenic effects reported from use of bromocriptine to achieve conception. The drug can be discontinued during pregnancy, then re-instituted after delivery. Patients with microprolactinomas desiring fertility can also be treated with transsphenoidal surgery. Surgical cure and subsequent fertility has been reported in 83% of patients (50). Surgery is also indicated for patients with macroadenomas with persistent mass effect despite maximal tolerated dose of bromocriptine; patients who are noncompliant, have serious side effects from bromocriptine, or do not want to continue medical therapy; and women with microprolactinomas desirous of pregnancy. Preoperative prolactin levels correlate both with tumor size and operative success: surgical cure is achieved in 70% of patients with preoperative prolactin levels of 200 to 500 ng/ml, and 14% of patients with prolactin levels over 1000 ng/ml (51).
Bromocriptine is useful for treatment of residual or recurrent prolactinomas in patients who are poor surgical candidates. One caveat is that bromocriptine induces tumor fibrosis, which makes resection difficult and reduces the surgical cure rate by approximately half (52, 53). Radiation therapy is effective at relieving symptoms and reducing prolactin levels. However, because of the effectiveness of surgery and pharmacotherapy for this disorder, radiation therapy is rarely indicated. It is reserved for symptomatic patients with recurrent or residual tumor who are not surgical candidates and are unable or unwilling to control their tumor using bromocriptine.
Growth Hormone-Secreting Adenomas
Excess growth hormone secretion produces not only the visibly obvious deformities of giganticism or acromegaly but also results in severe, systemic metabolic effects that result in a significant decrease in life expectancy. These include atherosclerosis, cardiomyopathy, cerebrovascular disease, respiratory disease, and diseases of the muscles, joints, and peripheral nerves. Thus, the goals for managing growth hormone-secreting adenomas are normalization of growth hormone and somatomedin C levels, in addition to elimination of mass effect.
Two agents have been used for medical therapy of growth hormone-secreting adenomas: bromocriptine and somatostatin. Bromocriptine decreases growth hormone production in 74% of patients, but normalizes levels in only 20% (54). Shrinkage occurs only in isolated cases (55) and requires higher doses of boromocriptine than those used for prolactinomas. These tumors probably represent mixed prolactin cell/growth hormone cell adenomas.
More recently, octreotide, a somatostatin analog, has demonstrated its effectiveness in suppressing growth hormones levels to below 5 mg/ml in 50% of patients, and less than 2 mg/ml in 25% of patients (56). However, this drug must be given by subcutaneous injection two to three times per day, making it suitable only for intelligent, highly motivated patients who can administer the injections themselves. Because of the inconvenience and morbidity of medical treatment, and the effectiveness of surgery in curing most of these patients (57, 58), pharmacologic therapy is reserved for patients who are poor surgical candidates or those with recurrent or residual disease who are unwilling or unable to undergo re-operation. Radiation therapy is reserved for patients in whom an endocrinologic cure cannot be achieved by surgery combined with medical therapy.
Adrenocorticotropic Hormone-Secreting Adenomas
In contrast to hyperprolactinomas, the endocrinopathy of Cushings disease is life threatening. When untreated, the 5-year mortality rate is 50%; thus, the goal of therapy must be endocrinologic cure. Because most corticotropic adenomas are microadenomas, radiologic diagnosis by gadolinium-enhanced MRI is achieved in only 40% to 60% of cases. When a pituitary mass is noted by MRI of the sella, or suggested by diagnostic petrosal sinus sampling in the absence of pulmonary or adrenal lesions on radiologic examination of the thorax and abdomen, transsphenoidal surgery is almost always the treatment of choice. Tumor noted on MRI is usually easily found and resected, while sparing the normal gland. Systematic exploration and sectioning of the gland is indicated to locate radiographically occult adenomas. Usually, several deep vertical and horizontal incisions are made in the gland to find the abnormal tissue. This technique is often successful. When it is not, a total hypophysectomy is usually recommended for older adults, from whom consent should be obtained for this eventuality before surgery. Children and young adults in whom no tumor is found are treated conservatively with medical management and reimaging before further surgery or radiosurgery. However, hypophysectomy is sometimes required by the severity of the endocrinopathy. Resection of the central “mucoid wedge” has also been reported (46, 59). Unilateral hypophysectomy is also an option in patients with occult ACTH-secreting adenomas that have been localized to one side by petrosal sinus sampling (35). Surgical cure has been reported in 90% of microadenomas and 65% of macroadenomas using this technique, with a recurrence rate of 8.5% (60). Successfully treated patients have had an immediate fall in blood cortisol levels with gradual resolution of cushingoid features.
In patients who are refractory to surgical cure, or who are poor operative candidates, pharmacologic treatment or radiation therapy can be used to achieve endocrinologic control. Medical therapy consists primarily of agents that pharmacologically block adrenal steroidogenesis. Ketoconazole is the most useful of these and has been used to stabilize severely ill patients as a preparation for surgery or radiation therapy (61). Other pharmacologic agents such as metyrapone (62), cyproheptadine, a serotonin ingibitor (63), aminoglutethemide, and mitotane (64) have been used to control serum cortisol levels with variable degrees of success.
The delayed effect of radiation therapy makes this modality inadequate as the primary treatment. However, radiation is reported to control disease in 50% to 70% of patients by 3 years (33, 65). This makes it useful as adjuvant treatment when combined with pharmacologic treatment in patients who are poor operative candidates or have persistent or recurrent disease. Bilateral adrenalectomy is immediately effective in curing hypercortisolism, but because of the severe metabolic consequences and the possibility of inducing Nelsons syndrome in patients with occult pituitary adenomas, this procedure is reserved as a last resort in most patients.
Although pituitary adenomas producing Nelsons syndrome are usually macroadenomas and easily visualized, they are characteristically invasive and difficult to cure. Normalization of ACTH and hyperpigmentation occur in less than half the cases (66). Although data is sparse, there is some suggestion that radiation therapy may be somewhat helpful in patients with Nelsons syndrome.
Thyrotropic Adenomas
Thyrotropic adenomas are the least common adenomas, accounting for less than 1% of all pituitary adenomas. They can be microadenomas but often are invasive macroadenomas, particularly in patients who have undergone thyroid ablation. Surgical excision is the treatment of choice and can be achieved in most microadenomas. The surgical cure rate for invasive macroadenomas is only about 40% (67). Octreotide has been reported to reduce TSH levels but does not significantly reduce mass effect, and adjuvant radiation therapy should be used in these cases.
Endocrinologically Inactive Adenomas
Endocrinologically inactive adenomas consist of null cell adenomas and gonadotropin-producing adenomas, which do not produce symptoms of excess endocrine secretion. These collectively account for 25% of all pituitary adenomas. Patients typically present with macroadenomas with mass effect, although they may present with symptoms of mild hyperprolactinemia due to stalk compression. Symptoms of hypopituitarism are often more evident. The goal of surgery is reduction of mass effect, and the treatment of choice is surgery. Although gross total resection is the surgeons intuitive goal, the fact that these tumors occur mainly in the elderly and are slow growing with a high incidence of cavernous sinus invasion suggest that therapy be individualized. The transsphenoidal approach is usually the procedure of choice, and near-total to gross-total resection can usually be achieved, although microscopic invasion is usually present (68). Vision improves or stabilizes in over 90% of these patients (69), and headache and cranial nerve palsies due to cavernous sinus compression often resolve. The patients presenting with panhypopituitarism seldom regain normal pituitary function (70), and they require long-term hormonal therapy. Bromocriptine may decrease prolactin levels through a direct effect on the hypothalamic-pituitary axis. However, given the benign nature of these neoplasms, their slow growth rate, and the success of re-operative surgery, the authors prefer conservative management, or “watchful waiting,” of any residual tumor. If the tumor grows, consideration is given to re-operation before instituting radiation therapy.
Giant Adenomas
The management of giant adenomas is both complex and controversial. These are usually a subset of endocrinologically inactive adenomas presenting with mass effect and obstructive hydrocephalus. Giant adenomas producing prolactin and other hormones have also been reported. Treatment is surgical, but these patients tend to do poorly with a high incidence of morbidity, and a 20% mortality rate (7, 71). The pathophysiology of this phenomenon is unclear, but it is thought to be due to exacerbated hydrocephalus, seemingly related either to tumor swelling after an incomplete resection, or due to a traction effect on the third ventricle. These tumors should be managed conservatively, with debulking through a transsphenoidal approach. Ventricuostomy can be done before or during surgery if hydroephalus is present. Complete resection is rarely required or achieved. Regional radiation therapy may be useful in poor operative candidates.
Apoplexy
Pituitary apoplexy is a clinical emergency requiring urgent surgical decompression of the sella and parasellar structures, usually through a transsphenoidal approach.
Pituitary Carcinoma
Pituitary carcinoma is rare, with less than 40 cases reported (72). They appear to arise from pituitary adenomas of all types, although endocrinologically active adenomas permeate. They typically present with multiple local recurces followed by metastatic dissemination to the subchnoid space or extraneuronal sites such as bone, liver, limp node, lung, or kidney. This is usually accompanied by escalation of histologic grade. The most symptomatic re is usually the sella, and the therapy is directed at control the primary neoplasm. Aggressive re-operation is used when adjuvant radiation therapy and pharmacologic therapy indicated by the characteristics of the original adenoma. In most cases, death from pituitary carcinoma results from a failure of local control with progressive local invasion, rather than from metastatic disease.
Tumors of the Posterior Pituitary
Granular cell tumors, or choristomas, are usually benign and asymptomatic, although malignant transformation has been reported. Symptomatic granular cell tumors most often present with visual disturbance, although hypopituitarism, hyperprolactinemia, precocious puberty, and acromegaly also occur. Diabetes insipidus is rare. Transsphenoidal surgery is usually indicated for diagnosis and resection. There is no evidence that these tumors are radiosensitive (13).
Management of metastatic lesions is more complex. Although most metastases to the neurohypophysis are asymptomatic, metastases to the stalk may present with hypopituitarism or mass effect (13). Treatment is palliative, and consists of surgery, radiation therapy, chemotherapy, or combinations of these modalities depending on the need for tissue confirmation, tumor type, and the medical condition of the patient. Usually, metastases are amenable to excision using the transsphenoidal approach if this is clinically indicated, and surgery can prolong survival.
Craniopharyngiomas
Craniopharyngiomas are among the most challenging tumors to treat. Although they are slow growing, benign, extraaxial neoplasms, some consider them malignant by virtue of location. Their tenacious attachment to pial vessels and critical neurovascular structures increases the morbidity of aggressive resection. Yet, the high recurrence rate and consequent morbidity after subtotal resection make this option equally distasteful.
The presenting symptoms depend on the size and location of the tumor. Craniopharyngiomas present with mass effect and symptoms of increased intracranial pressure in 80 % of adults. Endocrine dysfunction, including partial or complete hypopituitarism and diabetes insipidus, occurs in most patients of all ages, although it is usually not the presenting symptom.
Craniopharyngiomas are classified as endosellar, suprasellar, or intraventricular based on their position relative to the sella, chiasm, and third ventricle (73). Endosellar craniopharyngiomas are subdiaphragmatic and confined to the sella. They comprise 3% to 8% of craniopharyngiomas (74). Suprasellar craniopharyngiomas arise from the mid-portion of the stalk and typically enlarge beneath the third ventricle, elevating the third ventricular floor. Intraventricular craniopharyngiomas arise from the infundibulum. They invade the floor of the third ventricle and often extend into the interpeduncular fossa. The latter two types are considered retrochiasmatic craniopharyngiomas.
There is general agreement that surgery is the treatment of choice for craniopharyngiomas. However, the choice of approaches, the extent of resection, and the use of adjuvant radiation therapy remain controversial. The difficulty is that these tumors recur unless gross total resection is achieved, and many believe that the initial operation represents the only opportunity for cure. However, the tumor capsule is tenaciously attached to vital structures such as the optic chiasm, optic tract, infundibulum, pituitary, hypothalamus, third ventricle, and the vessels that supply these critical structures. Despite advances in microsurgical technique, aggressive resection of these densely adherent tumors has resulted in a high rate of neurovascular and endocrine complications. Even in experienced hands, the mortality rate for primary craniopharyngiomas has been reported to be as high as 8% to 15%, with varying degrees of visual compromise in 50% to 60% of patients, and 80% of patients require postoperative endocrine replacement (75, 76). This morbidity is thought to result from devascularization of critical structures due to aggressive resection of tumor capsule. Thus, it is preferable to leave densely adherent capsule attached to critical neurovascular structures rather than pursue more aggressive resection of tumor capsule. This, it is preferable to leave densely adherent capsule attached to critical neurovascular structures rather than pursue more aggressive resection that may lead to unacceptable morbidity.
The choice of surgical approach depends on the location, shape, and size of the tumor. Endosellar lesions with an enlarged sella and a prominent cystic component can be safely approached through the transsphenoidal route. Because of their subdiaphragmatic position, these tumors are not attached to the hypothalamus or optic chiasm. After draining the cystic component under direct vision, the intracapsular contents are removed with a grasping forceps and curettes. The capsule collapses and can be detached from the under surface of the diaphragma sellae. Normal preoperative pituitary function is a relative contraindication to the transsphenoidal approach because the gland is often situated anteriorly within the sella and damaged during tumor resection. The stalk can usually be preserved.
Suprasellar craniopharyngiomas are best resected using transcranial approaches. If hydrocephalus is present, ventricular drainage should be instituted before definitive therapy. The most useful approaches are the pterional and the subfrontal. Both have the advantage of allowing direct visualization of the optic nerve and chiasm. The pterional approach is the shortest and offers superior visualization of the retrochiasmatic region. The subfrontal approach provides better visualization of the optic pathways by way of the subchiasmatic pathway, as well as a midline route to the lamina terminalis. The opticocarotid pathway may also be useful. In addition, access to the sella can be achieved by removing the tuberculum sellae with a high-speed drill.
Intraventricular craniopharyngiomas are best approached by the subfrontal-lamina terminalis approach, the interhemispheric-transcallosal approach, the interhemispheric-transcallosal approach, or a combination of these. The subfrontal-lamina terminalis approach allows direct visualization of the chiasm and permits good access to the anterior third ventricle. However, this approach also carries the greatest risk of hypothalamic damage. The interhemispheric-transcallosal approach utilizes the foramina of Monro, which are usually enlarged due to hydrocephalus, to resect the intraventricular portion of the tumor. Alternately, if the foramina of Monro is not enlarged, the interforniceal approach may be used. Although the latter two approaches minimize the risk of devascularizing the hypothalamus, they carry the risk of bilateral forniceal damage and consequent memory impairment. Because these approaches give access only to intraventricular tumor, they are often combined with pterional or subfrontal approaches. The transcortical approach is associated with a high risk of hydrocephalus and postoperative seizures, in addition to the aforementioned risk of bilateral forniceal injury, and should be avoided except in cases of marked preoperative ventricular dilatation.
In all approaches, the presence of a subarachnoid plane facilitates complete resection and preservation of the normal vasculature. Electrocautery often disrupts this interface and should be avoided whenever possible. Although radiation therapy has been effective in reducing recurrence and increasing survival in some series with minimal morbidity (77), the risks of radiation therapy have prompted the authors to follow patients with serial examination and imaging studies, and individualize treatment of recurrence.
Meningiomas
Usually, parasellar meningiomas are large, and present predominantly with mass effect. Visual loss is the predominant symptom. Pituitary insufficiency is uncommon and usually mild. Because resection of parasellar meningiomas requires resection of the dura itself, an intradural approach is required for complete resection. The choice of approach I governed by the location and orientation of the lesion. Meningiomas of the tuberculum sellae, olfactory groove, and anterior clinoids are best approached through a subfrontal approach. Meningiomas of the medial sphenoid wing and cavernous sinus require a pterional approach, whereas retrochiasmal meningiomas are usually best resected using the subtemporal approach. The transsphenoidal approach may be indicated for diagnostic biopsy.
Chordomas
Chordomas of the sella typically present with symptoms of mass effect and hypopituitarism depending on their size and direction of growth. Occasional small tumors confined to the sella can be resected through the transsphenoidal approach alone. At the end of such an operation, the clivus should be drilled beyond the obvious tumor margin because of invasion within cancellous lumen. A tumor-free margin of well vascularized bone should be observed. The clival dura should be exposed and prolapse freely into the bony defect. However, most chordomas involve parasellar structures as well. Because gross total resection is associated with increased survival (16), such patients require a more extensive extradural or transcranial approach than can be achieved by the transsphenoidal approach alone. An extended transsphenoidal, transmaxillary, or transoral approach may be required to maximize exposure in these cases. There are some studies suggesting that adjuvant proton-beam radiation therapy may also prolong survival (78).
Nonneoplastic Cysts
Rathkes cleft cysts, mucoceles, and arachnoid cysts are the most common nonneoplastic cysts of the sella. They are usually asymptomatic. Occasionally, however, patients do present with symptoms of mass effect, hypopituitarism, or mild hyperprolactinemia due to stalk compression. Suprasellar extension may occasionally produce visual loss or hypothalamic dysfunction. Transsphenoidal decompression of the cyst, or marsupialization, without excision of the cyst wall is usually curative.
Inflammatory and Infectious Lesions
Inflammatory Lesions
Lymphocytic hypophysitis, Langerhans cell histiocytosis, giant cell granuloma, and sarcidosis are the most common inflammatory lesions of the sella turcica. These sellar lesions are often indistinguishable from pituitary adenoma on MRI. Transsphenoidal biopsy is required for diagnosis, but radical resection is rarely indicated. Lymphocytic hypophysitis typically presents with mass effect, symptoms of moderate hyperprolactinemia, and varying degrees of hypopituitarism, although diabetes insipidus is unusual. At surgery, the adenohypophysis is yellow, rubbery, and firm, and the inflammatory mass frequently extends into the suprasellar region. Patients are started on hormonal replacement ant treated with steroids.
Langerhans cell histiocytosis (histiocytosis X) of the sella usually presents with diabetes insipidus because of its preferential involvement of the stalk and posterior pituitary gland. Mild to moderate hypopituitarism may also be present. These lesions should be resected. Adjuvant radiation therapy has also been used (13).
Granulomatous lesions such as giant cell granuloma and sarcoidosis present with hypopituitarism and, occasionally, hyperprolactinemia. Sarcoidosis may also produce mass effect, diabetes insipidus, and even hypothalamic dysfunction. After biopsy to exclude infectious processes, they are treated conservatively with endocrine replacement, if required, and steroids.
Infectious Lesions
Abscesses of the pituitary typically present with symptoms of mass effect and moderate to severe hypopituitarism. In the setting of meningitis, this diagnosis should always be considered. The transsphenoidal approach is used to debulk and to obtain a diagnostic specimen for aerobic, anaerobic, acid-fat, and fungal cultures. Patients are then treated with the appropriate antibiotic, antituberculous, or antifungal agents.
Vascular Lesions
Aneurysms and carotid cavernous fistulas of the infraclinoid or cavernous carotid may present with a clinical picture indistinguishable from that of an endocrinologically inactive pituitary adenoma. Visual deficit, mild hyperprolactinemia, and hypopituitarism are common. Fortunately, the diagnosis is readily made by MRI, magnetic resonance angiography, or conventional angiography. The treatment of aneurysms and carotid-cavernous fistulas is beyond the scope of this article.
REFERENCES
- Renn WH, Rhoton Al Jr. Microsurgical anatomy of the sellar region. J Neurosurg 1975;43:288-298.
- Tindall GT, Barrow DL,eds. Disorders of the pituitary. St. Louis: Mosby, 1986.
- Lang R. Anatomy of the sellar region. In: Samii M. Draf W, eds. Surgery of the skull base: an interdisciplinary approach. Berlin: Springer-Verlag, 1989:27-35.
- Annegers JF, Coulam CB, Abboud CF, et al. Pituitary adenomas in Olmsted County, Minnesota, 1935-1977. Mayo Clin Proc 1978:53:641-643.
- Costello RT. Subclinical adenoma of the pituitary gland. Am J Pathol 1936;12:205-216.
- Hardy J. Transsphenoidal microsurgery of the normal and pathologic pituitary. Clin Neurosurg 1969;16:185-217.
- Symon L. Jakubowski J, Kendall B. Surgical treatment of giant pituitary adenomas. J Neurol Neurosurg Psychiatry 1979;42:973-982.
- Wilson CB. Neurosurgical management of large and invasive pituitary tumors. In: Tindall GT, Collins R, eds. Clinical management of pituitary disorders. New York: Raven Press, 1979:334-342.
- Ridgeway EC, Klibanski A, Ladenson PW, et al. Pure alpha-secreting adenomas. N Engl J Med 1981;304:1254-1259.
- Kovacs K, Horvath E. Tumors of the pituitary gland. In: Atlas of tumor pathology. 2nd series, fascicle 21. Washington, DC: Armed Forces Institute of Pathology, 1986.
- Scheithauer BW, Kovacs K, Laws ER Jr. The pathology of invasive pituitary tumors with special reference to their functional classification. J Neurosurg 1986;65:733-744.
- Luse SA, Kernohan JW. Granular cell tumors of the stalk and posterior lobe of the pituitary gland. Cancer 1955;8:616-622.
- Albrecht S, Bilbao JM, Kovacs K. Nonpituitary tumors of the sellar region. In: Melmed S, ed. The pituitary. New York: Blackwell Science, 1995:591-676.
- Canbus B, Akar N, Ciplak G, et al. Tumors of the sellar-parasellar region: analysis of 238 cases. In: Samii M, ed. Skull base surgery. Basel: Karger, 1992:317-320.
- Grisoli F, Vincentelli F, Raybaud C, et al. Intrasellar meningiomas. Surg Neurol 1983;20:36-41.
- Gay E, Sekhar LN, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neuro-surgery 1995;56:887-897.
- Mathews W, Wilson CB. Ectopic intrasellar chordoma. J Neurosurg 1974;40:260-263.
- Max MB, Deck MDF, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981;31:998-1002.
- Roessmann U, Kaufmann B, Friede RL. Metastatic lesions in the sella turcica and pituitary gland. Cancer 1970;25:478-480.
- Branch CL. Laws ER. Metastatic tumors of the sella trucica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 1987;65:469-474.
- Buchmann E. Schweisinger G. Hypophyse and Hamoblastosen. Zentralbl Neurochir 1979;40:35-40.
- Shanklin WM. On the presence of cysts in the human pituitary. Anat Rec 1949;104:379-407.
- Lee JH. Laws ER Jr, Guthrie BL. Et al. Lymphocytic hypophysitis: occurrence in two men. Neurosurgery 1994;34:159-163.
- Nishio S. Mizuno J. Barrow DL. Et al. Isolated histiocytosis X of the pituitary gland. Neurosurgery 1987;21:718-721.
- Taylon C, Duff TA. Giant cell granuloma involving the pituitary gland. J Neurosurg 1980;52:584-587.
- Stern BJ. Krumholtz A, Johns C. et al. Sarcoidosis and its neurological manifestations. Arch Neurol 1985;42:909-917.
- Berger SA, Edberg SC, David G. Infectious disease in the sella turcica. Rev Infect Dis 1986;8:747-755.
- Domingue JN, Wilson CB. Pituitary abscesses: report of seven cases and review of the literature. J Neurosurg 1977;46:601-608.
- Ramos-Gabatin A, Jordan RM. Rimary pituitary aspergillosis responding to transphenoidal surgery and combined therapy with amphotericin-B and 5-fluoro-cytosine: case report. J Neurosurg 1981;54:839-841.
- Del Brutto OH. Guevar J, Sotelo J. Intrasellar cysticercosis. J Neurosurg 1988;69:58-60.
- Osgen T, Bertan V, Kansu T. et al. Intrasellar hydatid dyst: case report. Neurosurgery 1984;60:647-648.
- Martin JB. Management of hypersecretory pituitary adenomas. Clin Neurosurg 1980;27:99-247.
- Orth DN. Liddle CW. Results of treatment in 108 patients with Cushings syndrome. N Engl J Med 1971;285:243.
- Wilson CB, Role of surgery in the management of pituitary tumors. Neurosurg Clin North Am 1990;1:139-159.
- Benoit R. Pearson-Murphy BE, Robert F. et al. Hyperthyroidism due to a pituitary TSH secreting tumor with amenorrhea-galactorrhea. Clin Endocrinol 1980:12:11-14.
- Melmed S. ed. The pituitary. New York: Blackwell Science. 1995.
- Ciric I. Mikhael M. Stafford T, Lawson L, Garces R. Transsphenoidal management of pituitary macroadenomas with long term follow-up results. J Neurosurg 1982; 59:395-401.
- Cohen AR, Cooper PR, Kupersmith MJ, et al. Visual recovery after transsphenoidal removal of pituitary adenomas. Neurosurgery 1985; 17:446-152.
- Nicola G. Transsphenoidal surgery for pituitary tumors with extrasellar extension. Progress in Neurological Surgery 1975;6:149-164.
- Symon L. Jakubowski J. Transcranial management of pituitary tumors with extrasellar extention. J Neurol Neurosurg Psychiatry 1979;42:123.
- Schloffer H. Erfulgreiche Operation eines Hypophysentumors suf Nasalm Wege. Wien Klin Wochamschar 1907;20:621.
- Weiss MH. Transnasal transsphenoidal approach. In: Apuzzo MJ, ed. Surgery of the third ventricle. Baltimore: Williams & Wilkins, 1987:476-494.
- Hardy J. Transsphenoidal approach to the sella. In: Wilson CD. Ed. Neurosurgical procedures. Baltimore: Williams & Wilkins, 1992:21-40.
- King WA, Becker DP. Transphenoidal resection of cavernous sinus tumors. In: Samii M. ed. Skull base surgery, Base: Karger, 1992:458-463.
- Chiari O. Uever eine Modification der Schlofferschen Operation von Tumoren der Hypophyse. Wien Klin Wochenschr 1912;25:5-6.
- Black PM, Zerevas NT. Surgical management of sellar and parasellar lesions. In: Schmidik R, Sweet T, eds. Operative neurosurgical techniques. 2nd ed. New York: Grune & Stratton. 1988:300-307.
- Lalwani AK, Kaplan MJ, Gutin PH. The transphenoethmoid approach to the sphenoid sinus and clivus. Neurosurgery 1992;31:1008-1014.
- Galfinos JG, Fitzpatrick BC, Smith LR, et al. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg 1995;83:197-205.
- Landolt AM. Prolactinomas: preoperative bromocriptine treatment. Perspectives in Neurologic Surgery 1990;1:105-115.
- Laws ER Jr. Fode NC, Randal RV, et al. Pregnancy following transsphenoidal resection of prolactin-secreting pituitary tumors. J Neurosurg 1983;58:685-688.
- Tindall GT. McLanahan CS. Christy JH. Transphenoidal microsurgery for pituitary tumors associated with hyperprolactinemia. J Neurosurg 1978;48:849-860.
- Landolt AM, Keller PJ, Froesch ER, et al. Bromocriptine: does it jeopardize the result of later surgery for prolactinomas? Lancet 1982;2:657-652.
- Landolt AM. Osterwalder V. Perivascular fibrosis in prolactinomas: is it increased by bromocriptine? J Clin Endocrinol Metab 1984;58:1179-1182.
- Besser GM, Wass JAH, Thorner MO. Bromocriptine in the medical management of acromegaly. Adv Biochem Psychopharmacol 1980;23:191-198.
- Oppizzi C, Luizzi A, Chiodini P, et al. Dopaminergic treatment of acromegaly: different effects of hormone secretion and tumor size. J Clin Endocrinol Metab 1984;58:988-992.
- Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly: a randomized multicenter study. Ann Intern Med 1992;117:711-718.
- Laws ER JR, Piepgras DG, Randall RV. Neurosurgical management of acromegaly: results in 82 patients treated between 1972 and 1977. J Neurosurg 1979;50:454-461.
- Laws ER Jr, Rnadall RV, Abboud CF. Surgical treatment of acromegaly: results in 140 patients. In: Givens J, ed. Hormone secreting pituitary tumors. Chicago: Year Book, 1982:225-228.
- Hardy J. Atlas of transsphenoidal microsurgery in pituitary tumors. New York: Igaku-Shoin, 1991.
- Salassa RM, Laws ER Jr, Carpenter RC, et al. Transsphenoidal removal of pituitary microadenoma in Cushings disease. Mayo Clin Proc 1978;53:24-28.
- Sonino N, Boscaro M, Merola G, et al. Prolonged treatment of Cushings disease by ketoconazole. J Clin Endocrinol Metab 1985;61:718-722.
- Jeffcoate WJ, Rees LH, Tomlin S, et al. Metyrapone in long-term management of Cushings disease. Br Med J 1977;2:215-217.
- Krieger DT, Amorosa L, Linick F. Cyproheptadine-induced remission of Cushings disease. N Engl J Med 1975;293:893-896.
- Luton JP, Mahoudeau JA, Bouchard P, et al. Treatment of Cushings disease by o,p-DDD: survery of 62 cases. N Engl J Med 1979;300:459-464.
- Edmonds MW, Simpson WJK, Meakin JW. External irradiation of the hypophysis for Cushings disease. Can Med Assoc J 1972;107:860-862.
- Thapar K, Smith M, Elliott E, et al. Corticotroph adenomas of the pituitary: long term results of operative treatment. Endocrine Pathology 1992;31:553-555.
- Thapar K, Laws ER Jr. Pituitary tumors. In: Laws ER Jr, ed. Brain tumors: basis for individual management. Philadelphia: JB Lippincott, 1995:759-773.
- Selman WR, Laws ER Jr, Scheithauer BW, et al. The occurrence of dural invasion in pituitary adenomas. J Neurosurg 1986;64:402-407.
- Trautmann JC, Laws ER JR. Visual status after transsphenoidal surgery at the Mayo Clinic, 1971-1982. Am J Ophthalmol 1983;96:200-208.
- Nelson AT JR, Tucker H St G. Becker DP. Residual anterior pituitary function following transsphenoidal resection of pituitary macroadenoma. J Neurosurg 1984;61:577-580.
- Decker RE, Chalif DJ. Progressive coma after the transphenoidal decompression of a pituitary adenoma with marked suprasellar extension. Neurosurgery 1991;28:154-158.
- Pernicone PJ, Scheithauer BW. Invasive pituitary adenomas and pituitary carcinomas. In: Lloyd RV.ed. Surgical pathology of the pituitary gland. Philadelphia: WB Saunders, 1993:121-136.
- Northfield DWC, Rathke-pouch tumors. Brain 1957;80:293-301.
- Litofsky NS, Levy ML, Apuzzo MLJ. Craniopharyngioma. In: Apuzzo MLJ, ed. Brain surgery: complication avoidance and management. New York: Churchill Livingstone, 1993:313-378.
- Yasargil MG, Curcic M, Kis M, et al. Total removal of craniopharyngiomas: approaches and long-term results in 144 patients. J Neurosurg 1990;73:3-11.
- Baskin DS, Wilson CP. Surgical management of craniopharyngiomas: a review of 74 cases. J Neurosurg 1986;65:22-27.
- Hug EB, Fitzek MM, Liebsch HJ, Muzenrider KJE. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys 1995;31:467-476.
- Austin-Seymour M. Munzenrider J, Goitein M, et al. Fractionated proton radiation therapy of chordoma and lowgrade chordosarcoma of the base of the skull. J Neurosurg 1989;70:13-17.



