News Articles February 2022
Written on 04 February 2022.
News Articles July 2022
Patient Story: Shifting Bite Leads to Diagnosis of Acromegaly
A story in the New York Times tells the story of a woman who kept having problems with her bite. The dentist filed her teeth but sent her for and MRI when the jaw shifted again. That’s when a technician saw the tumor on her pituitary. Read more:
The Role of Computer Facial Analysis in Diagnosing Acromegaly
A new study from China looks at the use of computer facial analysis and machine learning to diagnose acromegaly. Researchers found that certain facial characteristics which can be identified with facial analysis (widening of the jaw, an increase of length and breadth of the face, a nose that has widened and moved up) can predict “the morbid state” with high accuracy. They conclude that facial analysis shows promise in the early diagnosis of acromegaly. Read more:
Can Psychosis Be the First Sign of Cushing’s?
An article in Cushing’s Disease News looks at a case study from Saudi Arabia where a woman with psychosis, in the form of auditory hallucinations, delusions and compulsive eating, was eventually diagnosed with Cushing’s Disease. She underwent surgery for a microadenoma and eventually her symptoms eased. Read more:
Patient story: Optician Helps Diagnose a Pituitary Tumor
A story in the Mirror recounts the pituitary journey of a 21-year-old woman in the U.K. called Ellie Musgrove, who suffered from blurred vision and headaches. Her GP prescribed antibiotics and ibuprofen. When that didn’t help, she saw an optometrist, who noticed swelling, suspected a tumor and referred her to the hospital. By that evening she was in surgery to drain a buildup of fluid caused by a pituitary tumor. She was diagnosed with Addison’s disease. Read more:

Caption: Optometrist Aqeel Mahmood with patient Ellie Musgrove (Courtesy Ellie Musgrove)
PNA Highlights June 2022
PNA Highlights June 2022

The awareness that health is dependent upon habits that we control makes us the first generation in history that to a large extent determines its own destiny.
Jimmy Carter
PNA Spotlight: Dr. Adam Mamelak
This month the PNA Spotlight focuses on Dr. Adam Mamelak, a neurosurgeon and co-director of the Pituitary Center at Cedars Sinai Medical Center in Los Angeles. Dr. Mamelak earned his B.A. in Physics at Tuft University and earned his MD from Harvard Medical School. He did a surgical internship and then a residency at the University of California at San Francisco Medical Center. He did a fellowship at the Epilepsy Research Laboratory at UCSF, and another postdoctoral fellowship in neuroscience at the California Institute of Technology & Huntington Medical Research Institutes in Pasadena, California. Dr. Mamelak was kind enough to answer a series of questions from the PNA. His answers follow.
What inspired you to choose your career path?

PNA Medical Corner: Incipient Gigantism
This month the PNA Medical Corner highlights an article co-authored by Dr. Albert Beckers, a longtime member of the PNA. The study looks at the case of a 7-year-old boy with a pituitary tumor due to a gene mutation. They conclude that not all tumors of this type respond to pasireotide.
AACE Clin Case Rep
. 2021 Dec 16;8(3):119-123.
doi: 10.1016/j.aace.2021.12.003. eCollection May-Jun 2022.
• PMID: 35602875 PMCID: PMC9123570 DOI: 10.1016/j.aace.2021.12.003
Abstract
Background: Our objective was to describe the clinical course and treatment challenges in a very young patient with a pituitary adenoma due to a novel aryl hydrocarbon receptor-interacting protein (AIP) gene mutation, highlighting the limitations of somatostatin receptor immunohistochemistry to predict clinical responses to somatostatin analogs in acromegaly.
The Women’s Assessment Calendar
The Women’s Assessment Calendar is a tool that can be used for many situations and for every woman of menstruating age. We will provide examples of how this can help women understand their bodies better and communicate with their doctors more effectively as well. Mothers can use this as a training and communication tool with their teen daughters and women can learn about mild “normal” symptoms to those that need the follow-up of a specialty physician.
HOW TO UNDERSTAND AND COMPLETE THE CHART
First of all notice the “Symptom Rating Scale” at the very top of the page. The rating scale is scaled from 1 to 3 in order of severity of symptoms. If there are no symptoms there is no need to put anything in a box. A rating of 1 indicates a “mild” level of discomfort but not enough to interfere with daily activities. A rating of 2 indicates a “moderate” discomfort and/or pain intensity that does interrupt or disrupt normal daily life. Finally, a 3 rating means the woman is unable to perform her normal daily activities or routine such as going to work, caring for her children, unable to get out of bed etc. Ratings that are consistently (many days of the month) in the 3 level would indicate a need for a medical consult with a physician.
Next, note the numbers at the top of the chart right under the symptom rating scale. The “Calendar Date” goes from 1 to 15 on the first side of the page, and 16 to 31 on the back of the page. These correspond to the menstrual cycle days, not the days of the month. For example, day 1 is the first day of menstrual bleeding. Each symptom is then checked each day after (if symptoms exist) until the first day of bleeding the following month. At that point a new chart would be used, if so desired.
Next, possible physical and emotional symptoms are listed on the left side. For every symptoms that is observed a number from 1 to 3 would be then placed in the box that corresponds to the day of the cycle (not the day of the month). Healthy women’s menstrual cycles are typically around 28 days total but this varies slightly from woman to woman. One of the valuable uses of this chart is to track just how long your cycle really is (not just a guess). Gynecologists and other doctors ALWAYS ask women of menstruating age how long their cycle lasts and when their last period began. So it is important for young women to learn to keep an accurate record of their cycle (for doctor visits and other reasons).
It is also important to know that one of the values of such a tool is for you and your physician to be able to quickly observe any trends that may appear. For example, several “3” rating days around a certain time of each month may be related to ovulation, or expected drops or increases in hormone levels.
What about the Hormones listed at the bottom of the page?
We have included a section at the bottom of the chart for your doctors to use if they so desire. Listed are some of the hormones that help regulate a woman’s menstrual cycle and also need to be assessed if problems develop. Men and women have many hormones in our bodies that help facilitate the functioning of many physical functions. Hormones such as estrogen, progesterone, growth hormones, thyroid and more affect the menstrual cycle and also emotions, physical strength, appearance, height and much, much more.
HOW MOTHERS AND OTHERS WHO HELP GUIDE TEEN CAN USE THIS AS AN IMPORTANT TOOL
Moms, teachers, nurses and others who help teen girls navigate the first few years of menses can use this chart in several ways. Firstly, it is important for young girls, and all women, to truly understand just how important the menstrual cycle is to overall health! Just as most of us know that “normal” body temperature is around 98.6 degrees. So too is the significance of the menstrual cycle for women in the child-bearing years! Most of us know that a body temperature of 103 degrees indicates a problem, so we should also know that every woman’s cycle should be about the same length (time bleeding as well as total number of days between cycles). Variances in the length of bleeding and/or total length of the cycle indicate a need to talk with a physician. Of course as menstruation begins for girls things do not get off to an even rate for awhile. It is normal for the body to take a while to find its own unique rhythm.
Many girls have not been given much more than the most basic information in school so parents need to be able to provide follow-up. Also, schools do not typically provide education about the range of things that are not typical. Girls often silently presume their physical pain or emotionally intense moments are “normal” so they do not ask or speak-up. Embarrassment about menstruation can cause girls to suffer needlessly. Mothers and others who provide this chart can use this as a way to talk with their girls about all the possible signs that can sometimes accompany their cycles as well as the importance of then talking with their doctors about any concerning symptoms and/or trends.
This should not be something that scares or needlessly frightens young girls! It is important for them to learn how important and complex their bodies are but in a way that appreciates Mother Nature’s creativity and wonder! Also, since women’s reproductive organs are internal it is important for each woman to understand just how important it is to give their doctors as much helpful information as possible!
WHAT ALL WOMEN NEED TO KNOW
Many women were never given proper, adequate, or factual information about the workings of their own bodies. One of the reasons why Raginghormones.com was created was to provide medically and scientifically sound answers and resources. The Women’s Assessment Calendar was created by doctors in order to help them collect accurate data. Especially in an age where doctors are rushed each day with intense case loads it can be very helpful to come prepared to any medical appointment prepared with symptoms already documented.
WOMEN IN THE LATER PHASES OF THE MENSTRAL CYCLE
Some of the symptoms listed on this chart refer to hot flashes, night sweats and more. There are many myths and misconceptions about when and how the menstrual cycle comes to an end. Even some women in their younger years sometimes experience such symptoms. This may mean something very different depending upon the age of the woman so, again, it is important to keep accurate records and discuss such things with your doctor. What is considered “normal” may depend upon each individual woman. Menopause is a normal and natural phase of life. Symptoms typically thought of as relating to menopause can, however, also occur at other times in life and this can mean something different and may need medical attention.
QUESTIONS
Q: Do I need to keep using the Assessment Calendar forever?
A: No. Typically one or two months are adequate but your doctor may want you to keep longer records.
Q: If I have symptoms does that mean I am sick?
A: No. The symptom check list is just a guide for understanding your own body and a tool to give to your doctor if you have frequent and/or severe symptoms.
Q: If I have a “3” day should I be concerned?
A: The Assessment Calendar should be used more to show trends, not any individual day that may be intense. No one symptom or rating should be alarming.
Q: What if my doctor refuses to look at the data I have kept for several months?
A: It is important to develop a healthy way to communicate with any doctor. Many studies have shown that clear, accurate, respectful patient to doctor communication is one of the best signs for good medical care!
The Endocrine System
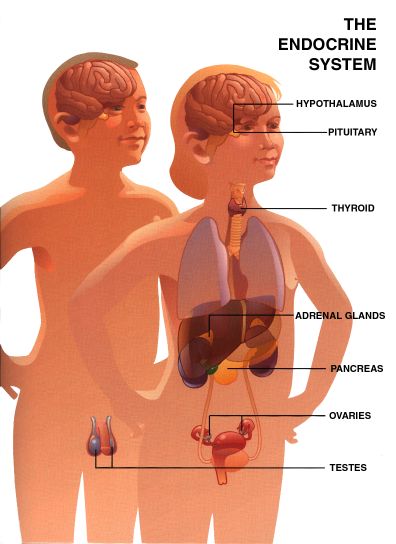
This section reprinted with permission from Pharmacia & Upjohn Company
Growth Hormone Use in Adults and Children
GH has been used to treat GH-deficient children for more than 35 years. Human GH originally was obtained from cadaver pituitaries and was available in only limited quantities. In 1985, however, data indicated that pituitary-derived GH was the likely source of contaminated material (prions) responsible for the development of Creutzfeldt-Jakobdisease (CJD) in three young men. CJD is a slowly developing, progressive, and fatal neurologic disorder. Consequently, production and distribution of pituitary GH for therapy were discontinued. CJD has now developed in more than 50 patients who received pituitary-derived GH.
Biosynthetic GH initially became available for prescription use in the United States in 1985. Human GH of recombinant DNA origin with an amino acid sequence identical to GH of pituitary origin has been produced commercially by several laboratories. Current GH preparations contain minimal impurities, are apparently safe, are readily available, and are in unlimited supply. These characteristics have led to expanded use of the hormone in both children and adults. At the time of this writing, GH has been approved by the US Food and Drug Administration (FDA) for treatment of GH deficiency (GHD) in both children and adults, short stature associated with chronic renal insufficiency (CR1) before renal transplantation, and short stature in patients with Turner syndrome. Recently, GH has also been approved for use in human immunodeficiency virus-associated wasting in adults. The abundant supply of GH in combination with recent scientific enthusiasm has prompted its use in various other conditions for which efficacy or safety data are not yet available from controlled clinical trials.
This report is based on a careful review of published data on the efficacy and side effects of GH therapy in children and adults. We have summarized herein the indications for GH use in children and adults, the conditions for which GH use has been investigated but not yet been approved, and the adverse effects of GH therapy. We believe that these guidelines will help clinical endocrinologists in treating patients with GH hut also realize that considerable controversy about its use will continue. Abbreviations used in this text are summarized in Table 1.
| Table 1: Explanations of Growth Hormone-Related Abbreviations | |
| AO | Adult onset |
| BIH | Benign intracranial hypertension |
| CJD | Creutzfeldt-Jakob disease |
| CO | Childhood onset |
| CRI | Chronic renal insufficiency |
| GH | Growth hormone |
| GHD | Growth hormone deficiency |
| GHRH | GH releasing hormone |
| IGF-I | Insulin-like growth factor-I |
| IGFBP-3 | Insulin-like growth factor binding protein-3 |
| ITT | Insulin tolerance test |
| IUGR | Intrauterine growth retardation |
| SCFE | Slipped capital femoral epiphysis |
Physiologic Effects of GH
GH promotes linear growth; the somatotropic effects occur partially through stimulation of the production of insulin-like growth factor-I (IGF-I).IGF-I produced primarily by the liver circulates throughout the body, whereas IGF-I produced in the growth cartilage acts locally as a paracrineautocrine growth factor. In addition, diverse metabolic actions of GH include its anabolic, lipolytic, and diabetogenic effects. GH is now shown to be produced throughout adult life and continues to have an important physiologic and metabolic role long after final height has been reached. The term somatopause has been used by some investigators to suggest that normal aging is associated with a gradual decline in GH secretion accompanied by a decrease in bone mass and lean body mass as well as an increase inadipose mass. Short-term administration of GH promotes lipolysis, stimulates protein synthesis, increases lean body mass, stimulates bone turnover, causes insulin antagonism, and alters total body water. The most dramatic metabolic effect of GH, however, is loss of visceral adipose tissue.
GH Therapy in Adults
The usefulness of GH treatment in adults who have completed their statural growth is based on the roles of GH in the following activities:
- Increasing bone density
- Increasing lean tissue
- Decreasing adipose tissue
- Bolstering cardiac contractility
- Improving mood and motivation
- Increasing exercise capacity
Another possible role for GH is the modulation of lipoprotein metabolism. GH decreases circulating levels of the atherogenic low-density lipoprotein; however, GH also increases circulating levels of Lp (a),which is atherogenic. Although evidence suggests that GH deficient patients are susceptible to development of premature cardiovascular disease, few data are available to demonstrate the ability of GH treatment to ameliorate this propensity. Furthermore, even though GH therapy seems beneficial, its cost-to-benefit ratio has yet to be determined, and few data are available on changes in rates of cardiovascular events, bone fractures, life span, or the incidence of malignant disease.
In the United States, an estimated total of 35,000 adults have GHD, and approximately 6,000 new cases of GHD occur each year.
Definition of Adult GHD
Severe GHD should be defined biochemically within an appropriate clinical context. In patients with hypothalamic-pituitary disease, the syndrome of GHD characteristically manifests with alterations in body composition, including abnormally low lean body mass and deficiencies in bone density particularly of trabecular bone as well as increased abdominal fat mass, which results in an increased waist-to-hip ratio. Muscle strength and exercise performance is often reduced. An impaired sense of well being and other psychologic complaints are common (Table 2). GHD must be distinguished from physiologically reduced GH secretion that occurs with aging. The benefits of GH supplementation in aging patients remain to be established and will not be considered in these guidelines.
| Table 2: Growth Hormone Deficiency in Adults: Cardinal Clinical Features |
| Increased weight and body fat mass; decreased lean body mass |
| Decreased exercise capacity |
| Decreased muscle mass and strength |
| Reduced cardiac performance |
| Reduced bone density and increased fracture rate |
| Poor sleep |
| Impaired sense of well-being |
An evaluation for GHD should be considered in all adult patients with adult-onset(AO) evidence of hypothalamic-pituitary disease, a history of cranial irradiation during either childhood or adulthood, or documented childhood-onset GHD (CO-GHD). This last factor is particularly important because some GH-deficient children are found to be GH sufficient in adulthood.
In patients with organic hypothalamic-pituitary disease, the likelihood of GHD increases with an increasing number of pituitary hormone deficits ranging from approximately 30% if only GH is deficient to almost 100% if three to four hormone deficiencies are present.
Assessment of patients with pituitary microadenomas for GHD may be unnecessary unless other pituitary hormone deficits are present or unless a strong clinical suspicion for GHD exists. All patients with idiopathic, isolated childhood GHD must be retested as adults before long-term GH replacement therapy is instituted.
Laboratory Diagnosis of GHD
GH testing can be performed in two ways that complement each other dynamic tests and biochemical markers.
Dynamic Tests of GH Secretion In most cases, the diagnosis of GHD in adult patients requires provocative testing of GH secretion. Random samples of GH are meaningless unless the levels are high, which is rarely found except in acromegaly. Care should be taken to ensure adequate hormone replacement for other hormonal deficiencies, such as thyroid, cortisol, and, when age appropriate, sex steroids.
In most academic centers, the insulin tolerance test (ITT) has been the validated study of choice. A 50% decrease in plasma glucose levels or a plasma glucose level of less than 40 mg/dL must be achieved for the test to provide meaningful results. Because the test has an inherent risk of profound hypoglycemia, the study should be performed with caution by an experienced staff under the supervision of a physician. The test is contraindicated in patients with abnormal electrocardiographic findings, with a history of ischemic heart disease or cerebrovascular disease, or with seizure disorders. If these safeguards are observed, the ITT is a safe clinical procedure when performed by experienced personnel. In general, the ITT is not recommended for patients older than 65 years of age.
In adults, the GH response to insulin-induced hypoglycemia is dependent on age, weight, and sex hormones, but most normal adults tested will have a peak GH secretion above 5 ng/mL (when GH is measured in a polyclonal competitive radioimmunoassay). Thus, values less than 5 ng/mL are considered indicative of GHD; the lower the value, the more severe the deficiency. In children and adolescents, in whom secretion maybe more robust and GH effects on growth may require higher levels of secretion than in older patients, values below 10 ng/mL are considered inadequate.
In patients in whom insulin-induced hypoglycemia is contraindicated (for example, those with seizure disorders) or unsafe or where appropriate testing arrangements are unavailable, alternatives to ITT should be used. Information is now emerging that intravenously administered arginine, either alone or in combination with GH-releasing hormone (GHRH), is useful. When only intravenously administered arginine is used, cut off values for a normal response are similar to those expected with ITT. When it is used in combination with GHRH, the response maybe augmented and the cutoff level is somewhat higher (9to 10 ng/mL). Tests that use glucagon, propranolol, or 1ev-odopahave a lesser-established diagnostic value in comparison with the ITT. Although useful as a diagnostic procedure in children, a test that uses clonidine is of dubious value for the diagnosis of GH deficiency in adults. In adults with the appropriate clinical history, generally only one provocative test of GH secretion is needed, although some investigators suggest that two tests should be done. Criteria for test results to confirm adult persistence of CO-GHD are not universally established. For reconfirmation of CO-GHD, generally only one stimulation test is recommended.
Biochemical Markers of GH Action Serum IGF-I concentrations are useful indicators of GH adequacy, and age-adjusted normal ranges are available. In adults, however, a normal serum IGF-I level does not exclude the presence of GHD. Conversely, in the presence of multiple pituitary hormonal deficiencies, especially in CO-GHD, a very low serum IGF-I indicates a high probability of GHD. Except for the patient with CO-GHD, the diagnosis of GHD should not, however, rely simply on IGF-I measurements but should be confirmed by provocative tests solely for GH secretion. Of note, the IGF-I concentration may also be reduced by poor nutrition, severe hepatic disease (storage diseases), poorly controlled diabetes mellitus and inadequately treated hypothyroidism. Measurements of IGF binding protein-3 (IGFBP-3) or the acid labile subunit of IGF-I have thus far not proved to offer any advantage over the measurement of IGF-I.
Treatment of GHD in Adults
All adults with substantiated GHD should be considered potential candidates for GH replacement therapy. The goal is to correct the abnormalities associated with GHD and to prevent the development of abnormalities consequent to long-term deficiency in adults. In patients with AO-GHD, the starting dosage should be very low (0.1 to 0.4 mg/day). This dose should be increased gradually on the basis of clinical and biochemical responses assessed at monthly intervals. A maintenance dosage rarely exceeds 1.0 mg/day in patients older than 35 years of age. Of note, this dose is substantially less than GH replacement doses in children and adolescents, in whom the dose is based on weight. Although the initial studies were performed with use of a per-kilogram dosing regimen, it has become apparent that obese patients were overdosed when body weight was used (even though they may require more hormone than lean patients, the amount needed is less than would be calculated on the basis of body weight), whereas women were underdosed (they may require more GH than do men if the close is calculated on a per-kilogram basis). In addition, elderly adults may require less hormone than middle-aged adults of the same bodyweight.
Therefore, therapy should begin with a low dose, and the dose should be gradually increased on the basis of clinical and biochemical responses. GH should be administered daily by subcutaneous self-injection, preferably in the evening. The best biochemical marker for GH is the IGF-I level in serum. Values of IGF-I should be maintained in the normal age- and sex-adjusted range. Because these ranges are laboratory- and assay-dependent, these data must be obtained from the reference laboratory used. IGFBP-3 alone has not been helpful, and other markers of GH action also require further validation. In the beginning, measurements of IGF-I for dose titration should be done approximately once a month. Subsequently. IGF-I levels should be assessed at least twice yearly.
Thyroid function should be monitored periodically by determining serum free thyroxine levels. Lipid levels should be measured at least annually. Simple anthropometric determinations, such as body weight and waist circumference, should also be recorded accurately and serially measured. Whole-body dual-energy x-ray absorptiometry, particularly of the lumbar spine with application of the reference standard available for the specific instrument used, should be performed in adult patients with long-standing adult GHD or high-dose glucocorticoid therapy. Blood glucose levels should also be monitored periodically.
GH therapy is not recommended during pregnancy in women with GHD because studies have not been conducted during pregnancy and placental GH is secreted from the end of the first trimester until term.
GH Therapy in Children
Currently in the United States, about 20,000 children receive GH therapy, and approximately 4,000 children are annually diagnosed as candidates for GH therapy. The US FDA has approved GH for use in the following pediatric conditions:
- Growth hormone deficiency
- Turner syndrome
- Chronic renal insufficiency
Growth Hormone Deficiency
GHD may result from abnormalities in the hypothalamus (most cases of idiopathic isolated GHD seem to result from deficient hypothalamic secretion of GHRH) or, less frequently, from pituitary pathologic conditions (such as pituitary tumors). Some causes are genetic (for example, abnormalities in the GH gene or in the PIT-i gene that regulates development of pituitary cells secreting GH, prolactin, luteinizing hormone, follicle-stimulating hormone, and thyrotropin), whereas others are acquired (such as tumors or Langerhans cell histiocytosis).
Several pitfalls in the diagnosis of GHD may be encountered. If thyroxine is deficient, then tests of GH secretion should be postponed until the thyroid deficiency is adequately replaced because GH secretion may be subnormal merely as a result of the hypothyroidism. If GHD is suspected in a peripubertal person with a growth pattern that resembles constitutional delay of growth and development, sex steroid priming before testing of GH secretion has been recommended by some investigators.
The cause of the GH insufficiency is particularly important in determining appropriate treatment. Because of its pronounced anabolic effects, GH is contraindicated in children with an active malignant condition. If GHD results from an intracranial tumor, absence of tumor growth or tumor recurrence should be documented for 6 to 12 months before initiation of GH treatment. Although GH treatment has not been demonstrated to induce growth of tumors, the theoretical possibility of such induction makes such a waiting period prudent.
GH treatment in children with CO-GHD is generally begun with a dosage of GH of 0.15to 0.3 mg/kg per week divided into daily or six times per week subcutaneous injections. Treatment is continued until final height or epiphyseal closure (or both) has been recorded. Continued treatment with GH into adulthood and beyond to achieve normal peak hone mass and optimize the metabolic effects of GH is now being evaluated (see GH Therapy in Adults).
Turner Syndrome
Turner syndrome, which occurs in 1 in every 2,000 live born girls, is due to abnormalities or absence of an X chromosome and is frequently associated with short stature, which may be ameliorated by GH treatment. Other features of Turner syndrome may include shortness of the neck and, at times, webbing of the neck, cubitus valgus, shortness of fourth and fifth metacarpals and metatarsals, a shield-shaped chest, and primary hypogonadism.
Because growth in height is variable in patients with Turner syndrome, the decision whether to treat with GH and the timing of such treatment should be made on the basis of each patients height and growth velocity. Often, treatment is initiated when a patients height declines below the 5th percentile or when the standard deviation score decreases to less than 2 standard deviations below the mean.
Treatment is often initiated with GH doses slightly higher than those used in treating GHD; a common starting dosage is 0.375 mg/kg per week divided into six or seven doses per week. Several studies suggest that statural growth may be optimized by concomitant treatment with oxandrolone in a daily dose of 0.0625 mg/kg.
Because patients with Turner syndrome most commonly have primary hypogonadism, treatment with estrogens may be necessary. Patients with Turner syndrome should participate in deciding on the timing of estrogen replacement. Delay of estrogen replacement beyond the normal age of puberty may help to optimize the outcome of GH treatment of short stature, but this delay must be weighed against the need for feminization. Initial estrogen replacement may be given in very low doses such as ethinyl estradiol, 50 to 100 ng/kg per day.
Chronic Renal Insufficiency
Growth delay in children with CR1 may result from numerous physiologic derangements, including acidosis, secondary hyperparathyroidism, malnutrition, or zinc deficiency. Before initiation of GH treatment in patients with CR1, existing metabolic derangements should be corrected. Major contributors to inadequate growth in children with CR1 are abnormalities in the GHIGF axis, resulting in low bioavailable IGF-I. In order to generate enough IGF-I to overcome these inhibitors, GH treatment is recommended at a dosage of 0.35mg/kg per week given six or seven times weekly.
Investigational Studies of GH Effectiveness
The following conditions are currently being actively studied relative to the efficacy of GH therapy.
Idiopathic Short Stature
Numerous clinical trials have documented the capacity of GH to induce growth acceleration in children with idiopathic, genetic, or primordial short stature. Several reports have indicated that GH treatment of non-GH-deficient short children does not increase adult height. Rather, after GH therapy initially causes growth acceleration, some reports suggest that it may enhance pubertal development and, occasionally, may shorten the duration of growth during puberty. In contrast, other studies have reported a gain of approximately 1 standard deviation(5 cm) in adult height over predicted adult height.
Constitutional Delay of Growth and Development
Constitutional delay of growth is characterized by normal prenatal growth followed by growth deceleration during infancy and childhood, which is reflected by declining height percentiles at this time. Between 3 years of age and late childhood, growth proceeds at a normal velocity. A period of pronounced growth deceleration may be observed immediately preceding the onset of puberty. Most notably, children with constitutional delay have later timing of puberty than do their peers. This delayed timing of puberty allows a longer period during which they are able to grow. Most commonly, these patients achieve normal adult height if no treatment is given.
At times, the combination of short stature accompanied and exaggerated by constitutional delay of growth and development in adolescents can cause sufficient psychosocial adolescent stress to warrant medical treatment. Although such therapy may consist of GH administered in the same doses used for treating GHD, other less costly treatments are available. In male patients, injectable testosterone may be administered in doses of 25 to 100 mg intramuscularly each month. Alternatively, anabolic” androgens such as oxandrolone maybe given in dosages of 0.0625 mg/kg per day. In female patients, such a delay occurs less frequently, and low doses of estrogens, as outlined for Turner syndrome, may be used. Of note, the use of GH or androgens will result in permanent closure of epiphyses, precluding further growth.
Intrauterine Growth Retardation and Russell-Silver Syndrome
Children within trauterine growth retardation (IUGR) or infants who are small for gestational age (a condition often called Russell-Silver syndrome) frequently show catch-up growth. Those children whose growth has not caught up by age 4 years may benefit from GH therapy, as some studies have suggested. Note that IUGR is heterogeneous in phenotype and in cause. Although patients with this condition have IUGR and may have relatively large heads with frontal bossing and triangular facies associated with micrognathia, not all cases are of the same etiologic origin. Distinguishing chromosomal, viral, and teratogenic causes before choosing therapy is important.
Skeletal Dysplasias
GH therapy has been tried in several skeletal dysplasias associated with short stature often in those cases associated with abnormal skeletal proportions. Much of the experience in treating these conditions has been gained in management of achondroplasia, a rhizomelic dwarfing condition due to mutations in the fibroblast growth factor receptor type III gene.
Although GH treatment of patients with achondroplasia has induced some growth acceleration, the growth velocities achieved have been insufficient to produce catch-up growth. Thus, the height of these patients is not altered in a major way so that it can approach the normal range for height. A limb-lengthening surgical procedure has been developed, which has succeeded in substantially increasing height in skeletal dysplasias. At present, such an operation may be associated with considerable discomfort. It may be complicated by infection, and it may necessitate prolonged and rigorous rehabilitation.
Osteogenesis Imperfecta
Osteogenesis imperfecta is caused by mutations in the gene for type I collagen. It is associated with bone demineralization and, in many instances, with retarded bone growth.
At times, osteogenesis imperfecta may be effectively treated with GH. In particular, patients may experience improved bone mineralization and improved growth with such treatment. Most commonly, GH tends to improve both growth and mineralization; in some patients, however, it is ineffective in improving either of these.
Prader-Willi Syndrome
Prader-Willi syndrome consists of hypothalamic obesity, short stature, developmental delay, hypogonadotropic hypogonadism, small hands and feet, and hypotonia. Most patients with this condition have deletion of portions of the paternal 15th chromosome (15q11-13). The hypothalamic disorder may result in impaired GH secretion in some patients, whereas the obesity, per se, may be responsible in others. Preliminary findings suggest that GH treatment in some patients with Prader-Willi syndrome accelerates growth, reduces hyperphagia, appreciably affects lipolysis, and decreases obesity.
Down Syndrome and Other Syndromes Associated With Short Stature and Malignant Diathesis
Because short stature is a characteristic of many syndromes, GH therapy has been attempted in several conditions, including Down syndrome, Falconi syndrome, and Bloom syndrome. The high basal risk of malignant tumor or leukemia in these syndromes, however, has led many pediatric endocrinologists to recommend that GH not he used because the occurrence of a malignant condition might then be linked (whether appropriately or not) to the GH.
Side Effects of GH Treatment
In the initial clinical trials composed predominantly of adults with AO-GHD, when starting doses of GH were higher than those now recommended, the most common side effects encountered during initiation of GH replacement therapy were fluid retention in conjunction with edema of the extremities, carpal tunnel syndrome, arthralgia, and myalgia. In a study of 115 adult patients with GH deficiency who were given GH replacement therapy for 6 months, edema developed in 37.4%, arthralgia in 19.1%, myalgia in 15.7%, paresthesias in 7.8%, and carpal tunnel syndrome in 1.7%. Of note, these symptoms most commonly occurred at the outset of therapy, and most resolved within1 to 2 months while therapy was continued.
Arthralgia, myalgia, and carpal tunnel syndrome are more frequent in adults but occur occasionally in GH-treated children. Peripheral edema is also more frequent in adults than in younger patients. Pseudotumor cerebri or benign intracranial hypertension (BJH), however, may occur more frequently in children. The US FDA has received reports of 23 cases of BIH associated with GH replacement, only 1 of which has been in an adult. In all cases, papilledema and symptoms of intracranial hypertension (for example, headaches) resolved after GH replacement therapy was stopped. Only a few of the patients who resumed GH therapy experienced recurrent headaches and papilledema.
Slipped capital femoral epiphysis (SCFE) may occur more frequently in children with GHD than in others. Whether GH indeed has this effect or whether this problem is merely the result of a diathesis induced by the condition of GHD, exacerbated by rapid growth, is uncertain. GH treatment has been suggested to increase the incidence of this problem further. If treated with GH, all children with knee or hip pain or limp should be carefully examined for SCFE. At times, lipoatrophy may occur in GH injection sites, but this finding is relatively uncommon. Some reports suggest that GH may increase creatinine levels in patients with end-stage renal disease. This phenomenon is more frequent in renal transplant recipients and may reflect increased risk of graft rejection.
In two large phase III prospective, randomized, placebo-controlled trials conducted in Europe, the effects of GH in critically ill intensive-care unit patients with acute catabolism have been studied. The inclusion criteria were ICU management after an open-heart surgical procedure, abdominal operation, multiple accidental trauma, or acute respiratory failure. The patients were given a dosage of 16 IU(5.3 mg) or 24 JU (8 mg) per day, dependent on body weight. The maximal treatment time was 21 days. The results of the two studies were similar and showed a significantly higher mortality in the GH-treated patients: 18.2% in placebo-treated patients and 41.7% in the GH-treated patients. Further assessment of the data, to develop a clear understanding of the reasons behind these differences, is ongoing. At this time, GH is not recommended for treatment of acute catabolism, including preoperative and postoperative treatment, critically ill patients, and burn patients. This recommendation does not apply to FDA-approved conditions.
GH induces transient resistance to the actions of insulin. In most patients, this action of GH increases circulating levels of insulin but not of glucose. In patients with limited insulin reserve, however, glucose intolerance may result. The GH effect on glycemia should also be monitored periodically by measurement of glycosylated hemoglobin levels. Several instances of pancreatis associated with GH therapy have been reported. The precise cause for this complication in GH treatment is uncertain.
Reports from Japan initially suggested an increased incidence of leukemia in GH treated patients. Careful studies in the United States have not confirmed an increased frequency of leukemia attributable to GH treatment. A major unanswered question is whether GH treatment further increases the incidence of leukemia in patients with other risk factors for leukemia (such as patients who have previously received radiation therapy).
The risk of certain malignant lesions particularly cancers of the gastrointestinal tract (that is, colon cancer) is increased in patients with acromegaly. It is, however, inappropriate to extrapolate from these findings that GH replacement in adults will have similar consequences. Currently established recommendations for prevention and early detection of cancer in the general population should be maintained and implemented in these patients as well. Continued regular follow-up with sensitive imaging techniques for residual pituitary or hypothalamic tumors should be part of any follow-up program. A baseline recent pretreatment imaging study is recommended before GH treatment is begun. GH may influence metabolism and action of many substances, including other hormones and medications. Alterations in the dose requirements of these compounds may be anticipated.
Transient gynecomastia has been described in children and adults during GH replacement therapy.
Overall, GH is contraindicated in patients with active malignant disease, BIH, and proliferative or preproliferative diabetic retinopathy. Potential for childbearing is not a contraindication, but GH therapy should be discontinued when pregnancy is confirmed. GH should not be used in critically ill patients in intensive-care units who have acute catabolism.
Conversion of European Dosing
Because most of the early studies on GHD treatment with GH in adult patients were done in Europe, publications cite dosing in IU or mU (international units), and early recommendations were often on a weight-adjusted(IU/kg) or square meter-adjusted (lU/in2) basis. More recently, studies have recommended beginning with single low closes in IU/clay (see Janssen et al.). The conversion of LU or mU to mg is3:1. Thus, a mean starting close of 0.6 LU is 0.2 mg/day. Mean maintenance dosages of 0.15 to 0.25 mU/kg per week are equivalent to0.05 to 0.08 mg/kg per week which for a 70-kg man would be 0.35 to0.56 mg/day (see Rosen et al.).
Specific Guidelines for GH Use in Adults and Children
Conditions for Which GH Therapy Is Recommended
Adults with GHD GH treatment of adults with GHD should be considered and has been associated with improved body composition, reduced body fat, and increased lean body mass. Patients with documented idiopathic GHD in childhood should be restudied. For the average 70-kg man, there commended dosage at the start of therapy is not more than 0.4 mg given as a daily subcutaneous injection. The dose may be increased, on the basis of individual patient requirements, to a maximum of 1.75 mg daily in patients younger than 35 years of age and to a maximum of 0.875 mg daily in patients older than 35 years of age. Lower doses may be needed to minimize the occurrence of adverse events in older or overweight patients. During therapy, the dosage should be decreased if side effects occur or IGF-I levels are excessive. The maintenance dose depends on the clinical and biochemical response. These doses should be altered to maintain circulating levels of IGF-I in the normal range for the patients age and sex. Serum free thyroxine and lipid levels should be assessed initially and at 6 to 12 months thereafter. Plasma glucose concentration is analyzed initially and every 3 months. Long-term treatment is being evaluated at this time.
Children with GHD GH treatment is indicated in children with documented GHD for correction of hypoglycemia and for induction of normal statural growth. If such patients are known to have had malignant tumors, remission should be substantiated for 6 to 12 months before initiation of GB treatment. A weekly dose of up to 0.30 mg/kg of body weight divided into daily subcutaneous injections is recommended. Periodic monitoring of thyroid function is indicated at approximately 6-month intervals. The appropriate time to discontinue GH treatment is controversial. Treatment for growth promotion should be continued at least until the handicap of short stature is ameliorated or until the patient is no longer responding to such treatment.
Turner Syndrome GH treatment is indicated for short girls with Turner syndrome. Patients may be treated with GH in starting doses of 0.375 mg/kg per week, usually divided into daily doses. Anabolic steroids, such as oxandrolone, may be used concomitantly in doses of 0.065 mg/kg per day with careful monitoring of bone maturation and of serum glucose levels. Estrogen replacement therapy should be discussed with each patient. If adolescent patients strongly believe that estrogen replacement is desirable, very low doses should be given (such as ethinyl estradiol, 50 ng/kg per day) until adequate growth has been achieved.
Chronic Renal Insufficiency In patients with end-stage renal disease and growth retardation. GH treatment may be considered once growth-inhibiting metabolic derangements (such as acidosis, secondary hyperparathyroidism, and under nutrition) are minimized. Treatment may he initiated with GH in a dosage of 0.35 mg/kg per week.
Conditions for Which GH Therapy Is Under Investigation
Miscellaneous Adult Conditions Limited but encouraging data are available about GH use in an array of conditions in adult patients (Table 3). GH therapy may help some patients in chronic catabolic states, older normal men and women, postoperative patients, those with states associated with excessive glucocorticoids, obese patients, and those within fertility. In most of these conditions, larger (pharmacologic) GH doses are needed to yield beneficial changes. For each of these conditions, however, the appropriate GH dose, regimen, and mechanism of action remain to be determined. Making definitive recommendations for the role of GH therapy in these states would be premature. Further data are needed on the use of GH in some conditions; for selected cases and for a limited time, GH therapy currently seems reasonable. Until more data are available, however, long-term GH therapy in these conditions is not recommended.
| Table 3: Growth Hormone Therapy in Adults |
| Approved Use |
|
| Experimental Use |
|
| *AIDS – acquired immunodeficiency syndrome |
Other Causes of Extreme Short Stature GH treatment may be considered for other children with extreme degrees of short stature. Attention should be paid to advancement of skeletal age because the effect of GH on adult height may be jeopardized by rapid skeletal age advancement. Some of these patients may be candidates for concurrent treatment with GH and with analogues of gonadotropin-releasing hormone if bone age advances rapidly and pubertal activity can be documented.
Constitutional Delay of Growth and Development Although GH treatment may be considered for children with extreme degrees of short stature associated with constitutional delay of growth and development, adolescent children with constitutional developmental delay may be treated more economically with low doses of androgen.
Prader-Willi Syndrome Children with Prader-Willi syndrome may benefit from GH therapy. Preliminary evidence shows GH related acceleration of statural growth, apparent reduction of appetite, and improved body composition. Additional studies will help determine whether this treatment is sufficiently likely to succeed in order to warrant widespread use.
Growth Retardation Due to Glucocorticoid Treatment GH treatment may be considered in extremely short persons with growth retardation attributable to glucocorticoid treatment. Before initiation of GH therapy in such patients, the glucocorticoid regimen should be reduced to the minimal dose needed to achieve a satisfactory clinical effect. If alternate-day glucocorticoid treatment is effective for management of a specific patients illness, that regimen should be introduced. Glucose intolerance should be monitored carefully. Finally, particular caution should be exercised in GH treatment of patients with conditions that may be exacerbated by such therapy. GH may, for example, exacerbate rejection in renal transplant recipients and may cause exacerbation in patients with diabetic retinopathy.
Clinical Practice of GH Therapy
GH therapy is best accomplished under the direct supervision of a clinical endocrinologist. Short-term GH treatment is safe in both children and adults. Continued monitoring of side effects and long-term treatment results is needed.
Optimal replacement dosages in adults have not been well defined; studies have suggested 0.1 to 1.0 mg/day. Considerable variability exists, however, in the appropriate GH dose for different patients and different conditions being treated. A single subcutaneous self-injection of GH into the abdomen, preferably in the evening, is best. The injection site should be rotated to minimize lipoatrophy. Daily administration is more effective in stimulating growth than injections three times per week. Although twice-daily GH schedules produce higher GH levels and may be superior to once-daily injections, inconvenience may compromise compliance.
Physiologic GH replacement must be distinguished from pharmacologic therapy. Replacement therapy of daily GH injections hardly simulates the normal, physiologic pulsatile pattern of GH secretion. Starting replacement therapy dosages for GH in children range from 0.02 to0.05 mg/kg per day and in adults from 0.00625 to 0.025 mg/kg per day. For a 70-kg man, the usual starting dose is 0.3 mg/day, with a maintenance dose of 0.35 to 0.56 mg/day or approximately 2 to 4 mg weekly of GH. Pharmacologic dosages in children are >1 mg/day and in adults >1 to 3 mg/day. The dosage should be increased slowly, on the basis of clinical as well as biochemical responses, and probably best at monthly intervals.
GH replacement maybe given throughout most of the lifetime of some affected patients. Physicians caring for these patients should be aware that dose requirements may decrease over time. Replacement therapy should be monitored carefully as the patient ages, and special emphasis should be placed on perceived and objectively measured benefits and side effects. If the patient receives no benefit, a withdrawal period should be considered. Because the diagnosis of GHD in adult patients, initiation of therapy, maintenance treatment, and monitoring of side effects are complex, these patients should remain under long-term surveillance by an endocrinologist experienced in treating pituitary-related disorders. Such a program of surveillance, which is the cornerstone of successful therapy, can be undertaken in partnership with an internist or family practitioner. Initial follow-up should be at monthly intervals. Thereafter, visits may be less frequent, yet should never be less than twice yearly. Because reimbursement for testing and treatment is often complex and time consuming, patient advocacy involves a considerable commitment. The practicing endocrinologist can assist the patient in achieving appropriate and lasting reimbursement.
GHRH has recently been approved for clinical use. In the future, smaller or orally administered molecules may be directly synthesizable rather than requiring use of recombinant DNA technology. These molecules maybe effective orally; however, they will work only when the patient has an intact pituitary.
The GH products approved for use in the United States are summarized in Table 4.
| Table 4: Growth Hormone Products Approved for Use in the United States | ||
| Product | Manufacturer | Indication |
| Protropin (somatrem) | Genentech | Pediatric GHD |
| Nutropin AQ and Nutropin (somatropin) | Genentech | Pediatric GHD, CRI, Turner syndrome, Adult GHD |
| Humatrope (somatropin) | Eli Lily | Pediatric GHD, Turner syndrome, Adult GHD |
| Norditropin (somatropin) | Novo Nordisk | Pediatric GHD |
| Genotropin (somatropin) | Pharmacia and Upjohn | Pediatric GHD, Adult GHD |
| Saizen (somatropin) | Serono | Pediatric GHD |
| Serostim (somatropin) | Serono | AIDS wasting |
| AIDS: Acquired immunodeficiency syndrome | ||
| CRI: Chronic renal insufficiency | ||
| GHD: Growth hormone deficiency | ||
Acknowledgment
We are indebted to Dr. Barbara Lippe for her review of and revision suggestions for these guidelines. Dr. Lippe is a Professor Emeritus of Pediatrics at the University of California/Los Angeles and is Senior Medical Director for the Pharmacia & Upjohn Company. She is a long standing AACE member.
References
- Arnato G. Carella C, Fazio 5, et al. Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab. 1993;77:1671-1676.
- Baum HBA, Biller BMK, Finkelstein JS, et al. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency: a randomized, placebo-controlled trial. Ann Intern Med. 1996;125:883-890.
- Bengtsson B-A, Eden 5, Lonn L, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309-317.
- Cook DJ, Greengold NL, Ellrodt AG, Weingarten SR. The relation between systematic reviews and practice guidelines. Ann Intern Med.1997;127:210-216.
- deBoer H, van der Veen EA. Why retest young adults with childhood-onset growth hormone deficiency? J Clin Endocrinol Metab.1997;82:2032-2036.
- Growth Hormone Research Society (GRS) Consensus Guidelines for Diagnosis and Treatment of Adults With GH Deficiency. Port Stephens Workshop, Australia, April 14-17, 1997.
- Guidelines for the use of growth hormone in children with short stature: a report by the Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endlocrine Society. J Pediatr. 1995;127:857-867.
- janssenYJH, Frolich M, Roelfsema F. A low starting dose of genotropin in growth hormone-deficient adults. j Clin Endocrinol Metab. 1997;82:129-135.
- Marcus R, Reaven GM. Growth hormone ready for prime time? J Clin Endlocrinol Metab. 1997;82:725-726.
- Meling TR, Nylen ES. Growth hormone deficiency in adults: a review. Am J MedSci.1996;311:153-166.
- Rosen T, Johannsson G, Johansson 1-0, Bengtsson B-A. Consequences of growth hormone deficiency in adults and the benefits and risks ofrecombinant human growth hormone treatment: a review paper. Horm Res. 1995;43:93-99.
- Vance ML. Growth hormone: nongrowth promoting uses in humans. Adv Endocrinol Metab.1992;3:259-269.
What is Acromegaly?
Acromegaly is an insidious disorder, due to chronic hypersecretion of growth hormone (GH) occurring in adulthood. GH hypersecretion prior to epiphyseal fusion results in gigantism. Elevated GH and/or insulin-like growth factor-1 (IGF-1) levels affect multiple organ systems resulting in acral and soft tissue growth, and metabolic dysfunction. Untreated acromegaly causes significant morbidity and mortality. Furthermore, mortality rates are twice the expected value, most commonly due to cardiovascular and respiratory complications. Control of GH hypersecretion lowers the morbidity and mortality associated with acromegaly. Ninety-fne percent of cases of acromegaly are caused by benign GH secreting pituitary adenomas; ectopic pituitary tumors and extrapituitary tumors (hypothalamus, pancreas, carcinoid, lung, ovary, breast) are found in a very small percentage of cases.
Treatment goals for patients harboring pituitary tumors include normalization of GH and IGF-1 levels, as well as removal of the adenoma with preservation of pituitary function and surrounding structures.
Therapeutic options include surgery, radiotherapy and drug therapy.
Approximately 30% of GH secreting pituitary adenomas are microadenomas (lOmm), resulting in persistence of GH hypersecretion postoperatively in half of the cases. Several patients, demonstrating postoperative “biochemical cure”, show recurrent tumor growth and increased GH levels when retested subsequently. Transsphenoidal pituitary tumor resection should be performed by surgeons experienced in this technique.
Radiotherapy is very effective in shrinking GH secreting pituitary tumors, but may take up to 20 years to lower GH levels and is associated with a high incidence of hypopituitarism. Data from longterm studies investigating the safety and efficacy of stereotactic radiosurgery (gamma knife) are not yet available.
Medical therapy includes dopamine agonists (DA), somatostatin analogs and GH antagonists. Only 15-20% of patients demonstrate GH suppression and 10% normalization of IGF-1 level on high doses of DA therapy. Somatostatin analogs normalize IGF-1 levels in 60% of patients. Somatostatin analogs are administered by subcutaneous injection in divided doses three to four tlmes daily. However, longacting somatostatin analog formulations administered intramuscularly once or twice a month will soon be available in the USA. Trovert, a pegylated analog of human growth hormone, functions as a growth hormone antagonist, displacing GH from hepatic receptors, thereby lowering circulating IGF- 1 levels. The safety and efficacy of a once daily subcutaneous injection of Trovert are currently being evaluated.
The addition of these long-acting and novel formulations to the treatment modalities for acromegaly greatly enhance therapeutic options.
References:
- Melmed S. Acromegaly. In: Melmed S., ed. The Pituitary. London Blackwell Science Inc., 1995: 413-442.
- Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. 1994. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinology (Oxf) 41:95-102.
- Fahlbusch R, Honegger J, Schot W, Buchfelder M. 1994 Results of surgery in acromegaly. In: Wass JAH ed. Treating acromegaly. Bristol: Journal of Endocrinology; 49-54.
- Laws Jr. ER, Carpenter SM, Scheithauer BW, Randall RV. 1987 Long term results of transsphenoidal surgery for the management of acromegaly. In: Robbins R, Melmed S, eds. Acromegaly: a century of scientific and clinical progress. New York: Plenum Press; 241-248.
- Eastman RC, Gorden P, Glatstein E, Roth J. 1992 Radiation Therapy of acromegaly. Endocrinol Metab Clin North Am 21: 693-712.
- Jaffe CA, Barkan AL. 1992 Treatment of acromegaly with dopamine agonists. Endocrinol Metab Clin North Amer 21:713-735.
- Ezzat S, Snyder PJ, Young WF et al. 1992. Octreotide treatment of acromegaly: a randomized multicenter study. Ann Intern Med. 117:711-718.
- Newman C, Melmed S, Snyder, PJ et al. 1995. Safety and efficacy of long term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients-a clinical research center study. J Clin Endocrinol Metab 80:1768-2775.
- Sassolas G. 1995. Medical Therapy with somatostatin analogs for acromegaly. Eur J Endocrinol 133:675-677.
- Cheung NW, Taylor L, Boyages SC. 1997 An audit of long term octreotide therapy for acromegaly. Aust NZ J Med. 27: 12-18.
- Harris AG. 1996. Treatment of acromegaly. In: Daly AF, ed. Acromegaly and its managment. Philadelphia: Lippincott-Raven; 49-68.
- Flagstad AK, Halse J, Rakke S, et al. 1997 Sandostatin LAR in acromegalic patients; long term treatment. J Clin Endocrinol Metab 80:3601-3607.
Transsphenoidal Surgery: Lesions of the Sella Turcica
Andrew E. Sloan, Keith A. Black, and Donald P. Becker
The treatment of sellar lesions is one of the more challenging and satisfying aspects of skull base surgery. The wide variety of lesions that occur in this region and the complexity of their treatment necessitates a thorough understanding of sellar anatomy, as well as the clinical symptoms and diagnostic evaluation of the myriad disease processes involving the pituitary gland.
ANATOMY
Sphenoid Bone and Sella Turcica
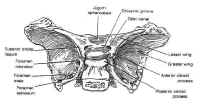
 The sphenoid bone is located at the base of the skull, posteroinferior to the anterior cranial fossa and anterior to the temporal and occipital bones (Fig. 1). The sella itself is a midline depression in the mid-portion of the sphenoid bone. It is bounded anteriorly and inferiorly by the sellar floor, anterolaterally by the anterior clinoid process, laterally by the cavernous sinuses, posteriorly by the dorsum sellae and the posterior clinoid processes, and superiorly by the diaphragmasellae. The floor of the sella is lined with an endocranial layer that is continuous with the diaphragma sellae superiorly, and contains the intercavernous or circular sinuses. These are highly variable, but typically develop in the anterosuperior and posterosuperior regions of the sella. The cavernous sinuses drain into the basilar venous plexus posterior to the dorsum sellae along the upper clivus, which joins the superior and inferiorpetrosal sinuses.
The sphenoid bone is located at the base of the skull, posteroinferior to the anterior cranial fossa and anterior to the temporal and occipital bones (Fig. 1). The sella itself is a midline depression in the mid-portion of the sphenoid bone. It is bounded anteriorly and inferiorly by the sellar floor, anterolaterally by the anterior clinoid process, laterally by the cavernous sinuses, posteriorly by the dorsum sellae and the posterior clinoid processes, and superiorly by the diaphragmasellae. The floor of the sella is lined with an endocranial layer that is continuous with the diaphragma sellae superiorly, and contains the intercavernous or circular sinuses. These are highly variable, but typically develop in the anterosuperior and posterosuperior regions of the sella. The cavernous sinuses drain into the basilar venous plexus posterior to the dorsum sellae along the upper clivus, which joins the superior and inferiorpetrosal sinuses.
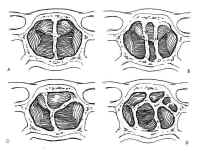
 The sphenoid sinus is just anterior and inferior to the sella, and is divided by one or more vertical septa that are often asymmetric (Fig.2). The degree of pneumatization of the sphenoid sinus varies among individuals and is classified as conchal, parasellar, or sellar (1,2) (Fig. 3). The sellar type is well pneumatized beneath the entire sella, which bulges into the sinus and is found in 70% to 86% of adults. The presellar sphenoid sinus extends only to the mid-portion of the sella, has no sphenoid bulges, and is found in 11% to 24% of adults. The conchal sphenoid sinus has minimal pneumatization andat least 10 mm of bone between the undeveloped sphenoid sinus and the sella turcica. This configuration is common in prepubertal children, but occurs in only 3% of adults.
The sphenoid sinus is just anterior and inferior to the sella, and is divided by one or more vertical septa that are often asymmetric (Fig.2). The degree of pneumatization of the sphenoid sinus varies among individuals and is classified as conchal, parasellar, or sellar (1,2) (Fig. 3). The sellar type is well pneumatized beneath the entire sella, which bulges into the sinus and is found in 70% to 86% of adults. The presellar sphenoid sinus extends only to the mid-portion of the sella, has no sphenoid bulges, and is found in 11% to 24% of adults. The conchal sphenoid sinus has minimal pneumatization andat least 10 mm of bone between the undeveloped sphenoid sinus and the sella turcica. This configuration is common in prepubertal children, but occurs in only 3% of adults.
Nasal Cavity
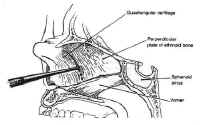 The nasal cavity provides access to the sphenoid sinus for transsphenoidal approaches. The most important anatomic features for the surgeon are the medial structures comprising the anterior septum that support the nose. These consist of the septal cartilage ventrally and superiorly, the vomer posteriorly and inferiorly, and the perpendicular plate of the ethmoid (Fig. 4).
The nasal cavity provides access to the sphenoid sinus for transsphenoidal approaches. The most important anatomic features for the surgeon are the medial structures comprising the anterior septum that support the nose. These consist of the septal cartilage ventrally and superiorly, the vomer posteriorly and inferiorly, and the perpendicular plate of the ethmoid (Fig. 4).
Pituitary Gland and Hypothalamus
The pituitary gland resides in the sella turcica and is attached tothe hypothalamus by the pituitary stalk. The stalk enters thesella through an opening in the diaphragma sellae, a membrane ofbasilar dura stretched between the tuberculum sellae and theposterior clinoids that separates the cranium from the sella. Asmall outpouching of the arachnoid accompanies the stalk throughthe central opening in the diaphragma in about 50% of specimens,to form a small pituitary cistern anterosuperior to theadenohypophysis (3). This is a potential source of cerebrospinalfluid (CSF) leak in the transsphenoidal approach to sellarlesions.
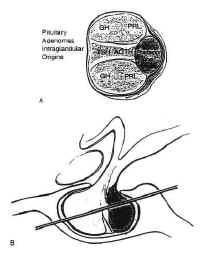 Figure 5The pituitary is composed of two lobes,distinct in embryologic origin, structure, and function, whichcome together during embryogenesis. The adenohypophysis isyellowish in color, and has a relatively firm consistency. Itarises from the primitive stomodeum as Rathkes pouch, whichgrows toward the neural tube to form the craniopharyngeal duct.The anterior portion of Rahtkes pouch develops into the parsdistalis, the functional portion of the anterior pituitary. A thinsleeve of tissue derived from the adenohypophysis extends upwardalong the pituitary stalk to form the pars tuberalis, which risesa short distance above the diaphragma sellae. The posterior wallof Rathkes pouch develops into the intermediate lobe, a cysticstructure, vestigial in function, lined by ciliated,mucus-producing adenohypophyseal cells. Although theadenohypophysis is uniform in structure, the cells aredifferentially concentrated in three regions. The cells in thecentral “mucoid wedge” produce thyroid-stimulatinghormone (TSH) anteriorly and adrenocorticotropic hormone (ACTH)posteriorly, and are basophilic (Fig. 5).
Figure 5The pituitary is composed of two lobes,distinct in embryologic origin, structure, and function, whichcome together during embryogenesis. The adenohypophysis isyellowish in color, and has a relatively firm consistency. Itarises from the primitive stomodeum as Rathkes pouch, whichgrows toward the neural tube to form the craniopharyngeal duct.The anterior portion of Rahtkes pouch develops into the parsdistalis, the functional portion of the anterior pituitary. A thinsleeve of tissue derived from the adenohypophysis extends upwardalong the pituitary stalk to form the pars tuberalis, which risesa short distance above the diaphragma sellae. The posterior wallof Rathkes pouch develops into the intermediate lobe, a cysticstructure, vestigial in function, lined by ciliated,mucus-producing adenohypophyseal cells. Although theadenohypophysis is uniform in structure, the cells aredifferentially concentrated in three regions. The cells in thecentral “mucoid wedge” produce thyroid-stimulatinghormone (TSH) anteriorly and adrenocorticotropic hormone (ACTH)posteriorly, and are basophilic (Fig. 5).
Some of these ACTH-producing cells invaginatethe posterior pituitary with increasing age, a phenomenon known as”basophilic invasion.” The cells of the lateral “acidophilicwings” produce primarily the peptide growth hormoneanteriorly and prolactin posteriorly. Gonadotropic cells producingluteinizing hormone and follicle-stimulating hormone are locateddiffusely throughout the gland.
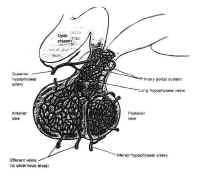 Figure 6The adenohypophysis is vascularizedpredominately by the superior hypophyseal artery by way of thehypophyseal portal system (Fig. 6). This venous plexus originatesin the anterior inferior portion of the hypothalamus and the floorof the third ventricle, where capillaries with a specializedfenestrated epithelium arise from branches of the superiorhypophyseal arteries. These capillaries are associated with theterminals of the tuberoinfundibular neurons, which synthesize thehypothalamic-releasing hormones and secrete them into thecapillary network. These vessels drain into the hypothalamicportal veins, which descend to the adenohypophysis along theanterior portion of the stalk, giving it a striated appearance. Inthe adenohypophysis, the portal veins develop into a secondcapillary network that supplies the secretory cells of theadenohypophysis and drains into the cavernous sinus.
Figure 6The adenohypophysis is vascularizedpredominately by the superior hypophyseal artery by way of thehypophyseal portal system (Fig. 6). This venous plexus originatesin the anterior inferior portion of the hypothalamus and the floorof the third ventricle, where capillaries with a specializedfenestrated epithelium arise from branches of the superiorhypophyseal arteries. These capillaries are associated with theterminals of the tuberoinfundibular neurons, which synthesize thehypothalamic-releasing hormones and secrete them into thecapillary network. These vessels drain into the hypothalamicportal veins, which descend to the adenohypophysis along theanterior portion of the stalk, giving it a striated appearance. Inthe adenohypophysis, the portal veins develop into a secondcapillary network that supplies the secretory cells of theadenohypophysis and drains into the cavernous sinus.
The neurohypophysis, or posterior lobe, appears gray in color and hasa soft, gelatinous consistency. It arises from the medianeminence, a downpouching in the floor of the third ventricle. Theupper portion becomes the pituitary stalk, whereas the distal endfuses anteriorly with the adenohypopjysis and remnants of thecraniopharyngeal duct and becomes the neurohypophysis. The stalkcomprises unmyelinated axons from the tuberoinfundibularneurosecretory neurons of the supraoptic and paraventricularnuclei of the hypothalamus, which transport granules ofvasopressin and oxytocin synthesized by these neurons to theposterior pituitary. These granules are stored in the nerveterminals of the neurohypophysis until released by actionpotentials generated in the cell bodies of the hypothalamicnuclei. The neurohypophysis is vascularized primarily by theinferior hypophyseal arteries, although the portal vessels alsoform some anastomoses with capillaries of the neurohypophysis.
Parasellar Structures
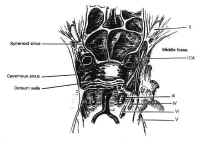 Figure 7Parasellar structures include the optic nerve and chiasm; the internalcarotid artery and its branches; the cavernous sinus and thecranial nerves contained within it; and the medial temporal lobes(Fig. 7). Symptoms and signs of sellar and parasellar lesions areoften the result of involvement of these structures. The opticchiasm overlies the diaphragm in about 80% of people. Theremainder are equally split between prefixed chiasms overlying thetuberculum sellae and postfixed chiasms overlying the dorsumsellae. The cavernous sinuses are lateral to the sella, andcontain the carotid artery and the sixth cranial nerve suspendedby fibrous trabecula. The third and fourth cranial nerves, and theophthalmic and maxillary divisions of the trigeminal nerve, liewithin the lateral walls of the cavernous sinus.
Figure 7Parasellar structures include the optic nerve and chiasm; the internalcarotid artery and its branches; the cavernous sinus and thecranial nerves contained within it; and the medial temporal lobes(Fig. 7). Symptoms and signs of sellar and parasellar lesions areoften the result of involvement of these structures. The opticchiasm overlies the diaphragm in about 80% of people. Theremainder are equally split between prefixed chiasms overlying thetuberculum sellae and postfixed chiasms overlying the dorsumsellae. The cavernous sinuses are lateral to the sella, andcontain the carotid artery and the sixth cranial nerve suspendedby fibrous trabecula. The third and fourth cranial nerves, and theophthalmic and maxillary divisions of the trigeminal nerve, liewithin the lateral walls of the cavernous sinus.
| Table 1. Differential diagnosis of sellar and parasellar masses | |
| Neoplastic lesions | Nonneoplastic lesions |
| Pituitary neoplasms | Cysts |
| Pituitary adenomas | Rathkes cleft cysts |
| Pituitary carcinomas | Mucoceles |
| Granular cell tumors | Arachnoid cysts |
| Nonpituitary neoplasms | Inflammatory and infections lesions |
| Craniopharyngiomas | Lympyhocytic hypophysitis |
| Meningiomas | Sarcoidosis |
| Chordomas | Giant cell granulomas |
| Dermoid cysts | Langerhans cell histiocytosis (histiocytosis X) |
| Epidermoid cysts | Abscesses |
| Germinomas | Vascular lesions |
| Teratomas | Aneurysms |
| Lipomas | Carotid cavernous fistulas |
| Melanomas | Cavernous angiomas |
| Metastases | |
PATHOLOGY
The close apposition of neural, endocrine, vascular, and meningeal tissue in the confined region of the sella gives rise to a large number of pathologic entities. Many of these are listed in Table 1. A complete pathologic description of each of these lesions, many of which are rare, is beyond the scope of this chapter. The pituitary adenoma and the craniopharyngioma, the most common sellar lesions, are discussed in detail. Other common neoplastic, cystic, inflammatory and infectious lesions are addressed only briefly.
Neoplastic Lesions
Pituitary Adenomas and Carcinomas
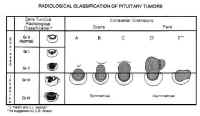 Figure 8Pituitary adenomas are the most common sellar neoplasm, accounting for 15% of all intracranial tumors. The annual incidence of these lesions is about 15 per 100,000 population (4). It is likely, however, that these symptomatic adenomas represent only a fraction of the true incidence of these neoplasms, because their prevalence in unselected autopsies is 22.5% (5). The highest incidence occurs in the third to sixth decades of life. Statistically, there appears to be an increased incidence in premenopausal women, though many contend that this is merely because of the sensitivity of the menstrual cycle to the relatively minimal endocrinologic imbalances often produced by these neoplasms. The symptoms produced in men are far less conspicuous and often ignored.
Figure 8Pituitary adenomas are the most common sellar neoplasm, accounting for 15% of all intracranial tumors. The annual incidence of these lesions is about 15 per 100,000 population (4). It is likely, however, that these symptomatic adenomas represent only a fraction of the true incidence of these neoplasms, because their prevalence in unselected autopsies is 22.5% (5). The highest incidence occurs in the third to sixth decades of life. Statistically, there appears to be an increased incidence in premenopausal women, though many contend that this is merely because of the sensitivity of the menstrual cycle to the relatively minimal endocrinologic imbalances often produced by these neoplasms. The symptoms produced in men are far less conspicuous and often ignored.
Pituitary adenomas have been classified by radiologic appearance, clinical presentation, and pathologic characteristics. The radiologic classification, known as the Hardy classification, distinguishes adenomas based on size and gross pathoanatomic relationships (6) (Fig. 8: Table 2). Adenomas 10 mm or less in diameter are classified as microadenomas: those greater than 10 mm as macroadenomas. Other investigators have classified adenomas greater or equal to 40 mm in diameter, or ascending to within 5 mm of the foramen of Monroe as giant adenomas (7). Although the original classification was based on lateral skull tomograms, these have been supplanted by computed tomography (CT) and magnetic resonance imaging (MRI) without formally altering the classification scheme.
| Table 2. Classification of pituitary adenomas | |||
| Radiologic | Anatomic | Surgical | |
| Sella Turcica | |||
| Grade 0 | Intact, normal contour | Micro | Enclosed |
| Grade I | Intact, focal bulging | ||
| Grade II | Intact, enlarged | ||
| Grade III | Destroyed, partially | ||
| Grade IV | Destroyed, totally | ||
| (Grade V) | (Distant spread through cerebrospinal fluid or blood |
||
| Extrasellar Extension | |||
| Suprasellar (symmetric) |
|||
| A. Suprasellar cistern B. Recesses third ventricle C. Whole anterior third ventrical |
|||
| Parasellar (asymetric) | |||
| D. Intracranial-intradural Anterior Midline Posterior E. Extracranial-Extradural (Lateral cavernous) |
|||
Microadenomas are classified as being grade 0 or I depending on the presence of sellar bulging. Macroadenomas are graded from II to IV based on the degree of sellar enlargement and destruction. Macroadenomas are further staged A to E according to the degree of suprasellar and extrasellar extension. This classification scheme has proven useful in surgical planning and correlates with operative risk and outcome (8).
adenomas can be classified clinically in accordance with symptomatic endocrinologic activity. Endocrinologically active adenomas are those that produce and release endocrinologically active anterior pituitary hormones such as prolactin, growth hormone, ACTH, and TSH. These typically present with symptoms of endocrinopathy. In contrast, endocrinologically inactive adenomas either fail to produce hormone (i.e., null cell adenomas); produce hormone that is inadequately secreted (i.e., a-subunit only [9]) or biologically inactive (i.e., silent corticotropic adenomas); or fail to produce symptoms (i.e., follicle-stimulating hormone or luteinizing hormone in male patients).
Pathologic grading schemes for pituitary adenomas originally used cytoplasmic staining affinities to distinguish tumor subtypes. More recent studies have demonstrated, however, that cytoplasmic staining affinities correlate not only with the contents of the secretory granules, but with their density. The classification scheme currently in use uses immunohistochemistry, supported in some cases by ultrastructural morphology, to classify adenomas into 10 types based on the storage and secretion of granules filled with pituitary hormones (10) (Table 3).
| Table 3. Pathologic classification of pituitary adenomas |
| Prolactin cell adenomas |
| Growth hormone cell adenomas |
| Mixed prolactin cell-growth hormone cell adenomas |
| Acidophilic stem cell adenomas |
| Corticotropic cell adenomas |
| Gonadotropic cell adenomas |
| Thyrotropic cell adenomas |
| Plurihormonal adenomas |
| Null cell adenomas |
The invasiveness of adenomas has been classified radiographically, surgically, and histologically. Although radiologic and gross surgical invasiveness appear to correlate with poor outcome, there appears to be no correlation between microscopic dural invasion, ploidy, mitotic index, and the aggressiveness of these neoplasms, which occasionally invade locally into bone, vessels, dura, or the brain (11).
Carcinoma of the pituitary is extremely rare, and impossible to diagnose based on histologic features alone. It is thought to arise from pituitary adenomas of all types and is defined as Hardy grade V by distant spread through blood or CSF. These tumors typically present with multiple local recurrences followed by metastatic dissemination to the subarachnoid space or extraneuronal sites such as bone, liver, lymph node, lung, or kidney.
Primary Neoplasms of the Posterior and Intermediate Lobes of the Pituitary
The neurohypophysis is rarely the site of clinically significant primary neoplasms. The most common of these is the granular cell tumor, also referred to as choristoma. The histogenesis of these lesions is uncertain, but they are found in 6.8% to 17% of pituitary glands of asymptomatic people at autopsy (12), and may also occur in the stalk. They most often present in the fifth decade of life. Gliomas, gangliocytomas, and hamartomas of the neurohypophysis are extremely rare (13).
Craniopharyngiomas
Craniopharyngiomas comprise 1% to 3% of intracranial tumors. They most commonly present as suprasellar masses, although approximately 20% are primarily intrasellar, and they are the most frequent parasellar lesions of children and adolescents and the second most common sellar lesions in adults. They are thought to be derived from cell rests of the intermediate lobe that arise from remnants of Rathkes pouch and are typically filled with a viscous fluid, rich in lipid and cholesterol, grossly resembling motor oil. They are frequently calcified and surrounded by intense gliotic reaction.
Meningiomas
Meningiomas are the third most common sellar lesions, comprising 1% to 3% of lesions in this region (14). Peak incidence of meningiomas is between 40 and 50 years of age, and women outnumber men by fourfold or more. Purely intrasellar meningiomas are rare (15). More commonly, meningiomas arising at the tuberculum sellae, medical sphenoid wing, olfactory groove, diaphragma sellae, or cavernous sinus invade the sellar and parasellar structures.
Chordomas
Chordomas are slowly growing, expansile, extradural neoplasms thought to arise from notochordal remnants. Median age at presentation is 45 years, and there is a slight male predominance. About 40% arise in the skull base, and of these, 33% involve the sellar or parasellar region (16). These tumors typically originate in the clivus and erode through the posterior clinoids and dorsum sellae into the sella and sphenoid sinus. Primary sellar lesions also occur (16, 17). Metastatic dissemination is a late occurrence in 10% to 20% of cases.
Other Primary Neoplasms
Gliomas of the optic nerve and chiasm, germ cell tumors, and hamartomas also occur in the parasellar region and should be considered in the differential diagnosis of sellar lesions, particularly in children. Epidermoid and dermoid cysts comprise approximately 1% and 0.1%, respectively, of all intracranial neoplasms, and occasionally present as sellar lesions in adults.
Metastatic Neoplasms of the Sella
The sella is a common site of systemic metastasis. Gross evidence of sellar metastasis occurs in 1% to 10% of patients dying of systemic cancer (10), although microscopic analysis reveals an incidence approaching 27% (18, 19). However, sellar metastases usually occur in the context of advanced systemic disease and are rarely symptomatic (20). The most common manifestation of metastatic disease is osseous involvement of the sella. Metastatic deposits also occur in the pituitary gland and neurohypophysis, but two thirds of these result from contiguous spread. Hematopoietic metastases commonly arise as meningeal lesions, particularly in non-Hodgkins lymphoma (21).
Nonneoplastic Lesions
Cysts
Benign cysts of the pituitary are identified in 13% to 23% of routine autopsies (22). These include Rathkes cleft cysts, mucoceles, and arachnoid cleft cysts.
Rathkes cleft cysts are epithelial cysts derived from the remnants of Rahtkes pouch. Although the involution of the craniopharyngeal duct is usually accompanied by the involution of Rathkes pouch, persistent, discontinuous, cystic remnants of the intermediate lobe are common. These usually are not large enough to be symptomatic. Those that are, presumably grow by progressive accumulation of their colloidal content. They are typically lined with cuboidal epithelium, but cysts lined with squamous epithelium indistinguishable from craniopharyngioma have also been described. They present over a wide range of ages and have no gender predilection.
Mucoceles are epithelial cysts arising from the paranasal sinuses. They are histologically indistinguishable from Rathkes cleft cysts, and differentiation relies on anatomic evidence of extension from a paranasal sinus obtained radiologically or at surgery.
Arachnoid cysts consist of distended areas of arachnoid filled with CSF that occasionally become symptomatic because of mass effect. They can be sellar, suprasellar, or both. Their pathogenesis is unclear: some believe them to be congenital, whereas others contend they are acquired.
Inflammatory and Infectious Lesions
Inflammatory Lesions
The Most common inflammatory lesions of the pituitary are lymphocytic hypophysitis, Langerhans cell histiocytosis, giant cell granuloma, and sarcoidosis. Lymphocytic hypophysitis is a destructive inflammatory process affecting the anterior pituitary that is presumed to be an autoimmune phenomenon. It occurs mainly in women, either during pregnancy or in the first postpartum year, and is often associated with autoimmune diseases of other endocrine glands. It also occurs in older patients of both genders (23).
Langerhans cell histiocytosis, formerly known as histiocytosis X, consists of a group of poorly understood eosinophilic granulomatous disorders that may be localized or diffuse. Involvement of the central nervous system in patients with disseminated disease is not uncommon, although isolated lesions of the sella are extremely rare (13, 24). These granulomas appear to have a predilection for the stalk and adenohypophysis; the posterior pituitary is usually spared.
Giant cell granuloma and sarcoidosis are also granulomatous lesions. Giant cell granulomas are noncaseating granulomas of the adenohyp-ophysis and infundibular stalk. They are typically multiple and have neither a sexual predilection nor an association with pregnancy (25). Sarcoidosis of the central nervous system occurs in 5% of patients with systemic sarcoidosis. Although neuritis is the most common presentation, inflammation of both the anterior and posterior lobes of the pituitary, as well as the stalk and hypothalamus, also occurs in about 1% of patients (26).
Infectious Lesions
Infection of sellar structures occurs only rarely. Bacterial abscesses may arise hematogenously or by secondary extension from an anatomically contiguous focus. Acute sphenoid inusitis or osteomyelitis are the most likely niduses for contiguous spread. The most frequent organisms are Staphylococcus aureus, Streptococcus pneumoniae, group A Streptococcus, and Klebsiella (27). There seems to be a predilection or abscesses to occur in the bed of a preexisting sellar lesion such as pituitary adenoma or craniopharyngioma (28). Tuberculosis, still endemic to certain areas, typically involves the sella in a dense, basilar meningitis, with or without arthritis. Intrasellar tuberculomas are usually associated with systemic tuberculosis and can result in pituitary destruction. spergillosis may also present with an inflammatory sellar mass (29), as can parasitic diseases such as cysticercosis (30) or echinococcosis (31).
Vascular Lesions
Aneurysms of the sellar region usually derive from the infraclinoid or intracavernous carotid artery. They are readily diagnosed by MRI and mentioned here merely as a diagnostic consideration to be excluded.
Clinical Presentation
Patients with sellar lesions present with a myriad of symptoms and signs. These can be classified as general or specific.
General Symptoms and Signs
The general symptoms and signs produced by sellar lesions result from mass effect, which stretches or compresses the densely packed neurovascular structures of the sella. These structures include the internal carotid artery and its proximal branches; the optic nerve, chiasm, and tracts; the pituitary gland; the infundibular stalk; the hypothalamus; the cavernous sinus and the cranial nerves within; the frontal and medial temporal lobes; the pons and midbrain; and the ventricular system.
Visual Manifestations
The most common manifestation of sellar or parasellar lesions in adults is visual impairment. Chiasmal compression produces a deficit of the bilateral superior temporal visual quadrant initially, followed by involvement of the inferior temporal quadrant. A junctional scotoma consisting of ipsilateral central scotoma with a contralateral superior temporal quadrantanopsia may also be produced by involvement of optic nerve fibers crossing into the chiasm at acute angles (Von Willebrands knee). Further compression may lead to irreversible chiasmal damage, resulting in total blindness. Isolated compression of the prechiasmal optic nerve results in an afferent pupillary defect known as Marcus Gunns sign.
Hypothalamic and Pituitary Dysfunction
Compression of the hypothalamus may lead to dysregulation of pituitary secretion as well as a myriad of other physiologic processes regulated by the hypothalamus. These include water balance, body temperature, level of consciousness, and behavior.
Compression of the median eminence or infundibular stalk may result in the impairment of synthesis of hypophysiotropic hormones or their transport to the pituitary gland itself. This results in varying degrees of hypopituitarism as well as diabetes insipidus. By interfering with the transport of dopamine the predominant prolactin-inhibiting factor to the lactotrophs, stalk compression also produces a paradoxical mild to moderate hyperprolactinemia (typically 30 150 ng/ml). This may present as hypogonadism or galactorrhea in women or men.
Compression of the pituitary gland itself also commonly results in hypopituitarism. The clinical symptoms depend on the cell types affected, and the degree of hyposecretion. The gonadotrophs are the most vulnerable and are usually affected first, leading to amenorrhea or oligomenorrhea in women, gonadal atrophy in men, and loss of libido in both sexes. Thyrotrophs and somatotrophs are usually affected next. Corticotrophs have the greatest functional reserve, and addisonian symptoms of nausea, vomiting, and postural hypotension usually present only in severe panhypopituitarism.
Cavernous Sinus Involvement
Sellar lesions extending into the cavernous sinus may involve the third, fourth, and sixth cranial nerves, resulting in partial or complete ophthalmoplegia. This is more commonly found in meningiomas than pituitary adenomas or craniopharyngiomas. The oculomotor nerve is the most commonly affected nerve in the cavernous sinus, followed by the abducens and trochlear nerves. Involvement of the first two branches of the trigeminal nerve results in numbness or pain in these distributions.
Headache
Headache results from stretching or compressing the dura of the diaphragma sellae, tentorium, cavernous sinus, or the base of the skull, all of which are innervated by the first division of the trigeminal nerve. Other mechanisms by which sellar lesions produce headaches are inflammation or pressure on other pain-sensitive dural structures, and elevated intracranial pressure due to mass effect or obstructive hydrocephalus.
Hydrocephalus
Sellar lesions with significant suprasellar extension may obstruct the foramen of Monro, producing unilateral or bilateral obstructive hydrocephalus. This may result in headache, papilledema, and decreased levels of consciousness. Stretching of the sixth cranial nerve may also produce an abducens palsy.
Cortical Involvement
Sellar lesions with significant suprasellar or parasellar extension may compress adjacent brain. Usually, slow-growing lesions reach large size before becoming symptomatic. Compression of the frontal lobes produces personality change and memory loss, whereas temporal compression or irritation may induce seizure.
Apoplexy
Pituitary apoplexy is a clinical manifestation of hemorrhage or infarction of a pituitary adenoma. The syndrome is characterized by the acute onset of severe headache and meningismus, often accompanied by nausea and vomiting. Partial or complete ophthalmoplegia is common, and a decreased level of consciousness may also occur. This clinical emergency results from the acute onset of mass effect on parasellar structures such as the optic chiasm or the cavernous and appears to be more frequent in clinically non-functional macroadenomas.
Specific Symptoms and Signs
Specific symptoms and signs may be diagnostic of certain endocrinologically active pituitary adenomas.
Prolactinomas
Prolactinomas account for about 30% to 40% of all pituitary adenomas and are the most commonly encountered endocrinologically active adenomas. They present differently in men and women. Women present threefold more frequently than men and complain of oligomenorrhea or amenorrhea, often with infertility and galactorrhea. They are typically 20 to 30 years of age and have microadenomas with moderately elevated prolactin levels (>200 ng/ml). In contrast, men typically present between 40 and 50 years of age, with general symptoms of mass effect. Men often harbor invasive mactoadenomas with significantly higher prolactin levels. They often admit to decreased libido or impotence, but rarely present with this as a chief complaint. Galactorrhea also occurs occasionally in men.
Growth Hormone-Secreting Adenomas
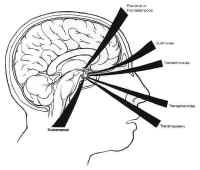 Figure 9: Surgical approaches to the sella turcicaExcess growth hormone due to pituitary adenoma results in giganticism if manifested in childhood before fusion of the epiphysis of the long bones. In adults, these tumors present with acromegaly, resulting in gross enlargement of soft tissues of the face and the extremities. Bone density also increases, resulting in degenerative joint disease, spinal stenosis, and compressive neuropathies. Acromegaly is also associated with systemic illness, including brittle diabetes, hypertension, severe atherosclerosis, and organomegaly. Poorly understood respiratory disease and cardiomyopathy result in decreased life expectancy for these patients.
Figure 9: Surgical approaches to the sella turcicaExcess growth hormone due to pituitary adenoma results in giganticism if manifested in childhood before fusion of the epiphysis of the long bones. In adults, these tumors present with acromegaly, resulting in gross enlargement of soft tissues of the face and the extremities. Bone density also increases, resulting in degenerative joint disease, spinal stenosis, and compressive neuropathies. Acromegaly is also associated with systemic illness, including brittle diabetes, hypertension, severe atherosclerosis, and organomegaly. Poorly understood respiratory disease and cardiomyopathy result in decreased life expectancy for these patients.
Adrenocorticotropic Hormone-Secreting Adenomas
Hypercortisolism, or Cushings syndrome, results in a myriad of clinical features, including moon facies, centripetal justments of head to facilitate improved visualization. Further flexion of the head improves access to the posterior sella and clivus, whereas extension improves exposure of the tuberculum and planum sphenoidale. The left thigh is positioned on a bolster to expose the superolateral aspect of the leg from the hip to the knee to facilitate a graft of fat and fascia lata, if needed. In very thin people, it may rarely be necessary to remove additional fat from the anterior abdominal wall. A lumbar subarachnoid drain may also be inserted before surgery for injection of saline or air to aid in bringing down a suprasellar mass. This has been used extensively by some surgeons (43) but is rarely done at the authors institution. Preoperative ventriculostomy may also be indicated for patients with hydrocephalus due to obstruction of the foramen of Monro or the third ventricle by a suprasellar mass. The fluoroscopy unit may also be used, although this is usually required only for patients with a conchal sphenoid sinus or patients lacking the usual anatomic landmarks, such as patients who have previously undergone transsphenoidal surgery or rhinoplasty.
 Figure 10: Position of the patient and operating team for the transnasal-transsphenoidal approachThe face and mouth are prepared with povidone-iodine from the bridge of the nose to the jaw to create a “semi-sterile” field. A vaginal pack is inserted in the oropharynx to prevent blood from entering the throat, and the gingiva and nasal mucosa are injected with 0.5% lidocaine with 1/200,000 epinephrine using a 25-gauge spinal needle until they blanch. The sphenopalatine ganglia and the ethmoid nerves are blocked with 1 x 3 cm cottonoids soaked with 4% cocaine to vasoconstrict the mucosa and shrink the nasal turbinates. Usually, the dissection is done on the left side for the convenience of the right-handed surgeon. However, if the septum is deviated significantly to the left, the right side should be used. Because the thigh is a sterile field, it is prepared separately. Two separate operative fields are maintained each with a separate set of instruments that are not to be shared.
Figure 10: Position of the patient and operating team for the transnasal-transsphenoidal approachThe face and mouth are prepared with povidone-iodine from the bridge of the nose to the jaw to create a “semi-sterile” field. A vaginal pack is inserted in the oropharynx to prevent blood from entering the throat, and the gingiva and nasal mucosa are injected with 0.5% lidocaine with 1/200,000 epinephrine using a 25-gauge spinal needle until they blanch. The sphenopalatine ganglia and the ethmoid nerves are blocked with 1 x 3 cm cottonoids soaked with 4% cocaine to vasoconstrict the mucosa and shrink the nasal turbinates. Usually, the dissection is done on the left side for the convenience of the right-handed surgeon. However, if the septum is deviated significantly to the left, the right side should be used. Because the thigh is a sterile field, it is prepared separately. Two separate operative fields are maintained each with a separate set of instruments that are not to be shared.
There are two variations of the transsphenoidal approach, the transseptal and the sublabial. The transseptal incision is quicker but gives a narrower exposure. It is useful when the nostril is large and the tumor is small. The sublabial incision, which the authors use exclusively, was introduced by Cushing and gives a wider exposure with better access to the superior aspect of the sella. Using a headlight for illumination, the gingival mucosa is incised from canine to canine down to the periosteum the maxilla (Fig. 11A). The mucoperiosteum is then dissected superiorly in a subperiosteal fashion to expose the pyriform aperture. The spine and any superomedial extension of the maxillary rostrum is then removed with a Kerrison rongeur as required to flatten the view of the floor of the nose (see Fig 11B).
 Figure 11: Transsphenoidal approach to the sella turcica. A: The gingival mucosa is incised from canine to canine with no. 15 blade. B: After dissection, the pyriform aperture is enlarged as required. C,D: The mucosa is elevated off the left side of the quadrangular cartilage ventral to the anterior boarder of the perpendicular plate. E: After freeing the quadrangular cartilage from the vomer and the perpendicular plate, the left anterior and inferior submucosal tunnels are sharply dissected, and the quadrangular cartilage is retracted to the right using the nasal speculum.Bilateral inferior tunnels are made first. The mucosa is first dissected from the nasal floor up to the maxillary crest of the septum using Cottle, Woodson, and Freer elevators. Next, the quadrangular cartilage is exposed sharply in the midline by incising the mucosa with a no. 15 blade. The subperiosteal dissection then proceeds posteriorly and superiorly to free the quadrangular cartilage from the interdigitation of the septal perichondrium with the periosteum of the maxillary crest (see Fig. 11C). These connections are typically concentrated along the junctions between the crest, the vomer, and the perpendicular plate of the ethmoid. The mucoperichondrium of the septal cartilage is extended beyond its bony septal attachments. This creates an anterior submucosal tunnel. The connective tissue bands connecting the quadrangular cartilage to the superior vomer and the anterior perpendicular plates are then sharply dissected with a Freer knife (see Fig. 11D). The quadrangular cartilage is then retracted laterally to the right with the nasal speculum (see Fig. 11E). The contralateral nasal mucosa and superior aspect of the quadrangular cartilage remain intact. The mucosa of the nasal floor is elevated into the inferior meatus on each side. The perpendicular plate is fractured, removed, and saved for possible use during closure. As the final septal mucoperiosteal dissection proceeds over the vomer to the posterior termination of the nasal septum, nasal specula of increasing length are used for the exposure. Care is taken to provide wide access to the sphenoid rostrum superiorly. By preserving a small, short spike of vomer or ethmoid plate protruding from the maxillary crest, a guidepost is maintained to the midline of the nasal cavity.
Figure 11: Transsphenoidal approach to the sella turcica. A: The gingival mucosa is incised from canine to canine with no. 15 blade. B: After dissection, the pyriform aperture is enlarged as required. C,D: The mucosa is elevated off the left side of the quadrangular cartilage ventral to the anterior boarder of the perpendicular plate. E: After freeing the quadrangular cartilage from the vomer and the perpendicular plate, the left anterior and inferior submucosal tunnels are sharply dissected, and the quadrangular cartilage is retracted to the right using the nasal speculum.Bilateral inferior tunnels are made first. The mucosa is first dissected from the nasal floor up to the maxillary crest of the septum using Cottle, Woodson, and Freer elevators. Next, the quadrangular cartilage is exposed sharply in the midline by incising the mucosa with a no. 15 blade. The subperiosteal dissection then proceeds posteriorly and superiorly to free the quadrangular cartilage from the interdigitation of the septal perichondrium with the periosteum of the maxillary crest (see Fig. 11C). These connections are typically concentrated along the junctions between the crest, the vomer, and the perpendicular plate of the ethmoid. The mucoperichondrium of the septal cartilage is extended beyond its bony septal attachments. This creates an anterior submucosal tunnel. The connective tissue bands connecting the quadrangular cartilage to the superior vomer and the anterior perpendicular plates are then sharply dissected with a Freer knife (see Fig. 11D). The quadrangular cartilage is then retracted laterally to the right with the nasal speculum (see Fig. 11E). The contralateral nasal mucosa and superior aspect of the quadrangular cartilage remain intact. The mucosa of the nasal floor is elevated into the inferior meatus on each side. The perpendicular plate is fractured, removed, and saved for possible use during closure. As the final septal mucoperiosteal dissection proceeds over the vomer to the posterior termination of the nasal septum, nasal specula of increasing length are used for the exposure. Care is taken to provide wide access to the sphenoid rostrum superiorly. By preserving a small, short spike of vomer or ethmoid plate protruding from the maxillary crest, a guidepost is maintained to the midline of the nasal cavity.
The long Cottle sepculum is now replaced by the Ha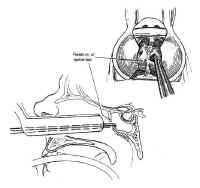 Figure 12: The sphenoid ostia are identified and enlarged using a rongeur. The entire rostrum of the sphenoid is removed in this fashion.rdy retractor. Careful dissection of mucoperiosteum over the face of the sphenoid is carried down to the sphenoid sinus ostia exposes bilaterally. The ostia are opened with a Kerrison rongeur until the sphenoid face begins to change its attitude from a vertical to a horizontal plane (Fig. 12). The speculum is continuously readjusted with the help of the “persuader” to capture the sphenoid face mucosa precisely under the tips of the retractor and to prevent “ballooning” of the mucosa into the visual field. Placement of the speculum in the sphenoid sinus should be avoided because fractures may result in traction damage to the optic nerve or the carotid artery. The entire anterior wall of the sphenoid sinus is removed. In patients with a conchal sphenoid sinus, a high-speed drill is used to open the sphenoid bone using fluoroscopic monitoring. Fluoroscopy may also be useful in other patients whose bony anatomy has been similarly obscured by previous transsphenoidal surgery or rhinoplasty. The sphenoid sinus mucosa is then removed using a pituitary rongeur or Decker.
Figure 12: The sphenoid ostia are identified and enlarged using a rongeur. The entire rostrum of the sphenoid is removed in this fashion.rdy retractor. Careful dissection of mucoperiosteum over the face of the sphenoid is carried down to the sphenoid sinus ostia exposes bilaterally. The ostia are opened with a Kerrison rongeur until the sphenoid face begins to change its attitude from a vertical to a horizontal plane (Fig. 12). The speculum is continuously readjusted with the help of the “persuader” to capture the sphenoid face mucosa precisely under the tips of the retractor and to prevent “ballooning” of the mucosa into the visual field. Placement of the speculum in the sphenoid sinus should be avoided because fractures may result in traction damage to the optic nerve or the carotid artery. The entire anterior wall of the sphenoid sinus is removed. In patients with a conchal sphenoid sinus, a high-speed drill is used to open the sphenoid bone using fluoroscopic monitoring. Fluoroscopy may also be useful in other patients whose bony anatomy has been similarly obscured by previous transsphenoidal surgery or rhinoplasty. The sphenoid sinus mucosa is then removed using a pituitary rongeur or Decker.
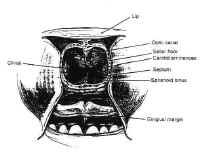 Figure 13The bulge of the sella is readily appreciated, as is the floor of the optic canal and the bony prominence of the carotid arteries (Fig. 13). Often, expansile sellar masses erode through the sellar floor, and resection can begin after merely opening the anterior wall of the sphenoid sinus. The carotid arteries may erode through the bone as well, however, particularly in older, hypertensive patients. When the sellar wall is intact, it is perforated and removed using a small osteotome and Kerrison rongeurs. The sphenoid septa are also removed when present. The bony resection can be extended superiorly to the tuberculum when there is a large degree of suprasellar extension.
Figure 13The bulge of the sella is readily appreciated, as is the floor of the optic canal and the bony prominence of the carotid arteries (Fig. 13). Often, expansile sellar masses erode through the sellar floor, and resection can begin after merely opening the anterior wall of the sphenoid sinus. The carotid arteries may erode through the bone as well, however, particularly in older, hypertensive patients. When the sellar wall is intact, it is perforated and removed using a small osteotome and Kerrison rongeurs. The sphenoid septa are also removed when present. The bony resection can be extended superiorly to the tuberculum when there is a large degree of suprasellar extension.
After the bony resection is complete, the dura is coagulated transversely with a bipolar cautery, then incised in a cruciate manner (Fig. 14). The intercavernous sinuses are often encountered at the superior and inferior margins of exposure, resulting in brisk bleeding. This is controlled by coagulating the leaves of the dura with a bipolar cautery.
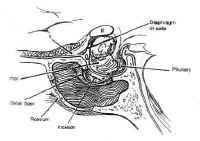 Figure 14: After removing the sellar floor with rongeurs, the dura is opened in a cruciate fashion.Once access to the sella has been achieved, the lesion can be sampled for biopsy and gently resected using Hardy microinstruments, bayonetted ring curettes, and aspiration. Pituitary adenomas are non-encapsulated, soft, and gelatinous, with the consistency of jam, and are readily removed using this technique. Great care is taken to preserve the normal gland, which is often seen as orange-tinged tissue abutting the stalk or the posterior wall of the sella. Cystic lesions, such as craniopharyngiomas, are drained with a needle under direct visualization before resecting the solid portion of the mass. Intermittent Valsalva maneuver by the anesthesiologist or injection of saline or air through a lumbar drain expedites tumor removal by elevating the intracranial pressure, which forces the mass inferiorly. The integrity of the arachnoid is maintained, when possible, to avoid a CSF fistula.
Figure 14: After removing the sellar floor with rongeurs, the dura is opened in a cruciate fashion.Once access to the sella has been achieved, the lesion can be sampled for biopsy and gently resected using Hardy microinstruments, bayonetted ring curettes, and aspiration. Pituitary adenomas are non-encapsulated, soft, and gelatinous, with the consistency of jam, and are readily removed using this technique. Great care is taken to preserve the normal gland, which is often seen as orange-tinged tissue abutting the stalk or the posterior wall of the sella. Cystic lesions, such as craniopharyngiomas, are drained with a needle under direct visualization before resecting the solid portion of the mass. Intermittent Valsalva maneuver by the anesthesiologist or injection of saline or air through a lumbar drain expedites tumor removal by elevating the intracranial pressure, which forces the mass inferiorly. The integrity of the arachnoid is maintained, when possible, to avoid a CSF fistula.
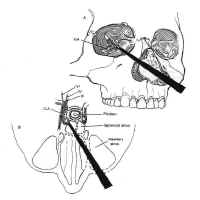 Figure 15: The extended transsphenoidal approach for lesions extending lateral to the carotid artery (Hardy grade D). The pyriform aperture is enlarged contralateral to the lesion, facilitating improved lateral exposure of the neoplasm.When lesions involve only the sella or the medial cavernous sinus, the standard transsphenoidal approach is sufficient. However, when lesions extend more laterally into the cavernous sinus (Hardy grade D), exposure can be improved by enlarging the pyriform aperture or performing a medial maxillotomy contralateral to the invaded cavernous sinus (44). This allows the speculum and the resection itself to be directed laterally (Fig. 15). The medial wall of the cavernous sinus consists of a single thick layer, contiguous with the lateral layer of sellar dura. Neoplasms invading the medial cavernous sinus typically obliterate the venous sinuses and can be removed using fine ring curettes and microdissectors. These instruments can be passed around the carotid artery to remove tumor that has extended laterally. Significant venous bleeding is usually not encountered until the patent portion of the cavernous sinus is exposed in the final phase of the resection, and is well controlled with Surgicel and direct pressure. Although this technique can often be used safely to resect neoplasm lateral to the carotid, narrowing of the caliber of the cavernous carotid on MRI suggests invasion of the vessel wall and is a relative contraindication to aggressive exploration using this technique.
Figure 15: The extended transsphenoidal approach for lesions extending lateral to the carotid artery (Hardy grade D). The pyriform aperture is enlarged contralateral to the lesion, facilitating improved lateral exposure of the neoplasm.When lesions involve only the sella or the medial cavernous sinus, the standard transsphenoidal approach is sufficient. However, when lesions extend more laterally into the cavernous sinus (Hardy grade D), exposure can be improved by enlarging the pyriform aperture or performing a medial maxillotomy contralateral to the invaded cavernous sinus (44). This allows the speculum and the resection itself to be directed laterally (Fig. 15). The medial wall of the cavernous sinus consists of a single thick layer, contiguous with the lateral layer of sellar dura. Neoplasms invading the medial cavernous sinus typically obliterate the venous sinuses and can be removed using fine ring curettes and microdissectors. These instruments can be passed around the carotid artery to remove tumor that has extended laterally. Significant venous bleeding is usually not encountered until the patent portion of the cavernous sinus is exposed in the final phase of the resection, and is well controlled with Surgicel and direct pressure. Although this technique can often be used safely to resect neoplasm lateral to the carotid, narrowing of the caliber of the cavernous carotid on MRI suggests invasion of the vessel wall and is a relative contraindication to aggressive exploration using this technique.
After maximal res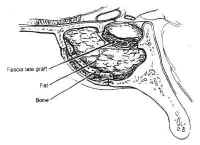 Figure 16: Repair of cerebrospinal fluid (CSF) fistula. If a CSF leak is identified during surgery, it may be patched with fascia lata and held in place with Gelfoam or fat, tamponaded with bone.ection has been completed, the sella and the pituitary gland itself are systematically inspected. If the tumor capsule prolapses into the sphenoid sinus, or there is a gross CSF leak visible, fascia lata, fat, and bone are used to reconstruct the sellar floor (Fig. 16). The fascia lata is placed in the sellar opening and sealed with fibrin glue after packing the sella with a small amount of fat or Surgicel. The fascia is then buttressed within the sella by the wedge of bony septum removed earlier. The integrity of the closure is then tested by having the anesthesiologist maintain a Valsalva maneuver at 40 mm Hg pressure. If the closure is satisfactory, the speculum is removed and the nasal mucosa is reapproximated and tamponaded using gauze impregnated with petroleum jelly. The gingival mucosa is reapproximated using 3-0 chromic suture, and a “mustache” dressing consisting of a folded gauze is placed below the nostrils. In cases with extensive CSF leaks, lumbar drainage is continued for 2 to 3 days. For large suprasellar lesions, the sella is packed regardless of whether a CSF leak is present. This is necessary because the resection may produce an “empty sella” secondarily. This may result in collapse of the diaphragma sellae and consequent traction on the chiasm, leading to decreased vision.
Figure 16: Repair of cerebrospinal fluid (CSF) fistula. If a CSF leak is identified during surgery, it may be patched with fascia lata and held in place with Gelfoam or fat, tamponaded with bone.ection has been completed, the sella and the pituitary gland itself are systematically inspected. If the tumor capsule prolapses into the sphenoid sinus, or there is a gross CSF leak visible, fascia lata, fat, and bone are used to reconstruct the sellar floor (Fig. 16). The fascia lata is placed in the sellar opening and sealed with fibrin glue after packing the sella with a small amount of fat or Surgicel. The fascia is then buttressed within the sella by the wedge of bony septum removed earlier. The integrity of the closure is then tested by having the anesthesiologist maintain a Valsalva maneuver at 40 mm Hg pressure. If the closure is satisfactory, the speculum is removed and the nasal mucosa is reapproximated and tamponaded using gauze impregnated with petroleum jelly. The gingival mucosa is reapproximated using 3-0 chromic suture, and a “mustache” dressing consisting of a folded gauze is placed below the nostrils. In cases with extensive CSF leaks, lumbar drainage is continued for 2 to 3 days. For large suprasellar lesions, the sella is packed regardless of whether a CSF leak is present. This is necessary because the resection may produce an “empty sella” secondarily. This may result in collapse of the diaphragma sellae and consequent traction on the chiasm, leading to decreased vision.
After surgery, patients are managed in the neurosurgery step-down unit, where close attention is paid to the ophthalmologic examination and the detection of diabetes insipidus. New deficits are rare, and improvement in the visual field examination is sometimes noted early in the postoperative period. Diabetes insipidus usually does not appear before 12 to 24 hours after surgery, and polyuria is not treated unless the diagnosis of diabetes insipidus is clear: urine output greater than 300 ml/hour for 2 consecutive hours with a specific gravity less than 1.005, urine osmolarity of 50 to 150 mOsm/L, and a serum Na of at least 145 and rising. The patient is allowed to drink ad libitum and treated with aqueous DDAVP (vasopressin), 1 ug every 12 to 24 hours intravenously or subcutaneously as needed. In the immediate postoperative period, serum sodium and osmolarity and urine output are monitored closely. The patients are encouraged to ambulate the night of surgery, and the Foley catheter is removed after 24 hours unless diabetes insipidus makes this inconvenient. Intravenous antibiotics are continued until the nasal packing is removed on the third postoperative day, after which the patient undergoes a postoperative MRI. Patients without complications are routinely sent home on the second or third postoperative day on a tapering dose of steroids, with follow-up appointments for both the neurosurgery and endocrinology clinics.
The most frequent complications of the transsphenoidal approach are CSF leaks and damage to the carotid artery or cranial nerves. CSF leaks can usually be avoided using the techniques outlined previously. Persistent leaks are usually controlled with prolonged lumbar drainage and elevation of the head. Re-operation for repair of a CSF leak refractory to conservative management is sometimes required and is usually done using the TNTS approach. Tension pneumocephalus, a related complication, occurs because of continual accumulation of intracranial air through a dural tear with a ball-value mechanism that prevents its egress. This is far more rare, but more deadly, and this possibility should be considered inpatients whose postoperative mental status deteriorates, particularly when a lumbar drain is in place. After the lumbar drainage is discontinued, and the diagnosis is confirmed, the patient is placed on 100% oxygen. The pneumocephalus gradually resolves over several days. Tension pneumocephalus may also be decompressed with a spinal needle transnasally or transcranially in emergent circumstances.
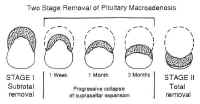 Figure 17: Two-stage removal of pituitary macroadenoma with suprasellar extension.Laceration of the intracavernous carotid is another rare but serious complication that occurs more frequently in cases requiring cavernous sinus exploration. Because of the narrowness of the field, temporary occlusion and primary repair of the carotid are impossible in the transsphenoidal approach. Lacerations are treated by holding direct pressure with Surgicel and cotton pledgets until the bleeding stops. An intra operative angiogram should be obtained when possible to document patency of the carotid and rule out contrast extravasation. Follow-up angiograms should be obtained before discharge and at 3 months to rule out pseudoaneurysms. Cranial nerve palsies as well as lesions of the brainstem and hypothalamus are also possible, although rare. After ruling out hematoma or pneumocephalus, they are managed expectantly. Recovery is variable.
Figure 17: Two-stage removal of pituitary macroadenoma with suprasellar extension.Laceration of the intracavernous carotid is another rare but serious complication that occurs more frequently in cases requiring cavernous sinus exploration. Because of the narrowness of the field, temporary occlusion and primary repair of the carotid are impossible in the transsphenoidal approach. Lacerations are treated by holding direct pressure with Surgicel and cotton pledgets until the bleeding stops. An intra operative angiogram should be obtained when possible to document patency of the carotid and rule out contrast extravasation. Follow-up angiograms should be obtained before discharge and at 3 months to rule out pseudoaneurysms. Cranial nerve palsies as well as lesions of the brainstem and hypothalamus are also possible, although rare. After ruling out hematoma or pneumocephalus, they are managed expectantly. Recovery is variable.
Because of the versatility and low morbidity of the TNTS approach, it can be used even in the presence of extensive suprasellar extension. When the lesion is soft (as pituitary adenomas usually are), the suprasellar mass often collapses into the sella, where it can be safely resected at the initial procedure. Occasionally, when the tumor does not descend immediately, the residual neoplasm may collapse further into the sella with time (Fig. 17), allowing the residual to be resected by repeat TNTS if further surgery is indicated.
Facial Degloving and Extended Maxillotomy Approaches
Lesions invading the superior clivus above the hard palate and medial to the carotid arteries, such as chordomas, medial chondrosarcomas, and juvenile angiofibromas can be approached using facial degloving or extended maxillotomy approaches either alone or in combination.
Transsphenoethmoidal Approach
The transsphenoethmoidal approach to the sella was introduced by Chiari (45). The advantage of this approach over the transsphenoidal approach is the shorter operative distance to the sella (46). However, it requires a facial incision, provides a narrower axial exposure, and carries a risk of injury to the optic nerve. One version combines this with medial maxillectomy for improved axial exposure of the sella (47). However, this combination holds little benefit over maxillotomy with midface degloving, which has a lower risk and superior cosmesis.
A 3-cm incision is made along the medial border of the nose, beginning from the inferior margin of the eyebrow, and the lacrimal sac is retracted laterally. The anterior ethmoid artery is sacrificed, and the lamina papyracea over the lacrimal fossa is removed, as is the underlying middle turbinate. The ethmoid mucosa is removed to identify the sphenoid ostia. The anterior and posterior walls of the sphenoid are then removed to expose the sella. The operation then proceeds as in the standard TNTS approach.
Transcranial-Intradural Approaches
The indications for transcranial-intradural approaches to the sella consist primarily of patients for whom transsphenoidal surgery is contraindicated for reasons discussed previously. Lesions invading the cavernous sinus laterally, with secondary sellar invasion; lesions that frankly violate the dura; and residual suprasellar or lateral cavernous sinus lesions that could have been only partially resected from the transsphenoidal approach are additional indications for transcranial approaches. In addition, repair of CSF leaks may also require a transcranial approach.
There are several possible transcranial approaches to the sella trucica, but the most useful are the transbasal, pterional, and subtemporal approaches (see Fig. 9). The choice of approach is determined by the location of the lesion with respect to the sella, and to some extent by the personal preference of the surgeon. These approaches are discussed briefly in the following sections specifically in terms of their utility for the resection of sellar and parasellar lesions.
Transbasal Approach
 Figure 18: The subfrontal approach to the sella turcica. The tuberculum sellae is removed with a high speed drill to facilitate exposure of a suprasellar mass.The transbasal approaches to the sella are versions of the anterior subcranial approach. A unilateral or bilateral frontal craniotomy is performed with or without orbitofrontal craniotomy. The tuberculum sellae is removed anterior to the chiasm with a high-speed drill (Fig. 18). This minimizes the effect of a prefixed chiasm, when present, and gives the surgeon an unobstructed view of the sella between the optic nerves with minimal brain retraction. In contrast to the other transcranial approaches, the pituitary stalk is easily identified and avoided, as are the carotid arteries and their branches. This approach is particularly useful for extradural lesions such as esthesioneuroblastomas and craniopharyngiomas, as well as for tuberculum sellae and olfactory groove meningiomas with secondary sellar involvement. There is a high risk of anosmia due to avulsion as the olfactory nerves with this approach, as well as a small risk of optic nerve or carotid injury. Frontal lobe swelling can usually be avoided by proper positioning, lumbar drainage, and minimizing brain retraction.
Figure 18: The subfrontal approach to the sella turcica. The tuberculum sellae is removed with a high speed drill to facilitate exposure of a suprasellar mass.The transbasal approaches to the sella are versions of the anterior subcranial approach. A unilateral or bilateral frontal craniotomy is performed with or without orbitofrontal craniotomy. The tuberculum sellae is removed anterior to the chiasm with a high-speed drill (Fig. 18). This minimizes the effect of a prefixed chiasm, when present, and gives the surgeon an unobstructed view of the sella between the optic nerves with minimal brain retraction. In contrast to the other transcranial approaches, the pituitary stalk is easily identified and avoided, as are the carotid arteries and their branches. This approach is particularly useful for extradural lesions such as esthesioneuroblastomas and craniopharyngiomas, as well as for tuberculum sellae and olfactory groove meningiomas with secondary sellar involvement. There is a high risk of anosmia due to avulsion as the olfactory nerves with this approach, as well as a small risk of optic nerve or carotid injury. Frontal lobe swelling can usually be avoided by proper positioning, lumbar drainage, and minimizing brain retraction.
Pterional Approach
The pterional approa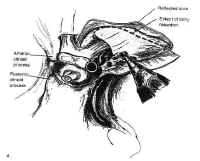 Figure 19: The pterional approach to sellar lesions. The anterior clinoid process can be removed intradurally with a high speed drill to facilitate exposure of the lateral parasellar region.ch is the shortest route to the parasellar region. This is usually done on the side of either the greatest tumor extension, or the patients worst vision. If neither of these are considerations, the craniotomy is done on the nondominant side. After performing a standard pterional craniotomy, the sphenoid ridge lateral to the superior orbital ssure is removed extradurally with a high-speed drill. The sylvian fissure is widely split and the frontal and temporal lobes are gently retracted to maximize exposure of the sellar region, and the anterior clinoid process is drilled away (Fig. 19). This gives excellent exposure of lateral parasellar lesions such as meningiomas or eccentric pituitary macroadeomas spilling into the middle fossa. Access to the sella itself, however, is somewhat impeded by the optic nerve, and carotid artery, which lie in the surgeons line of sight. In addition, the contralateral optic nerve may be poorly visualized. The risks of this approach include damage to the carotid artery as well as the optic and third cranial nerves.
Figure 19: The pterional approach to sellar lesions. The anterior clinoid process can be removed intradurally with a high speed drill to facilitate exposure of the lateral parasellar region.ch is the shortest route to the parasellar region. This is usually done on the side of either the greatest tumor extension, or the patients worst vision. If neither of these are considerations, the craniotomy is done on the nondominant side. After performing a standard pterional craniotomy, the sphenoid ridge lateral to the superior orbital ssure is removed extradurally with a high-speed drill. The sylvian fissure is widely split and the frontal and temporal lobes are gently retracted to maximize exposure of the sellar region, and the anterior clinoid process is drilled away (Fig. 19). This gives excellent exposure of lateral parasellar lesions such as meningiomas or eccentric pituitary macroadeomas spilling into the middle fossa. Access to the sella itself, however, is somewhat impeded by the optic nerve, and carotid artery, which lie in the surgeons line of sight. In addition, the contralateral optic nerve may be poorly visualized. The risks of this approach include damage to the carotid artery as well as the optic and third cranial nerves.
Subtemporal Approach
The subtemporal approach gives superior access to the posterior aspect of the sella, including the posterior clinoid process and the top of the clivus. It is most useful for lesions with retrochiasmatic extension such as chordomas and chondrosarcomas of the uppermost clivus, as well as meningiomas in that region. The approach begins with the standard pterional craniotomy. Aggressive temporal bone resection is used to minimize brain retraction. A zygomatic osteotomy may further enhance exposure. After opening the dura, the sylvian fissure is widely split, and the median temporal lobe is retracted laterally to reach the upper clivus and posterior clinoid process. The disadvantage of this approach is that the ipsilateral third and fourth cranial nerves are interposed between the surgeons line of sight and the sella. In addition, visualization of the contralateral posterior cerebral artery (PCA) and superior cerebellar artery (SCA) is poor. The major risk of this approach is temporal lobe damage due to excessive retraction.
The aforementioned three approaches can also be combined.
Adjunctive Surgical Techniques: Frameless Stereotaxy
The authors have also employed frameless stereotactic devices extensively for complex cases. This has been useful for intraoperative determination of the trajectory required for resection of certain skull base lesions, allowing the exposure to be optimized. It is also useful for intraoperative confirmation of the limits of resection and for determining the proximity of critical neurovascular structures in regions with distorted anatomy. Although brain “shift” after the dura is opened (48) limits the accuracy of this technique for deep intraparenchymal lesions, this technique is accurate for localizing skull base lesions.
TREATMENT
Pituitary Adenomas
The goals of treatment for patients with pituitary adenomas are to remove the mass effect (if present) and to normalize or restore hormonal function. Endocrinologically inactive microadenomas are usually discovered incidentally and rarely require treatment. The treatment of endocrinologically active microadenomas depends on their endocrine activity. Pharmacologic therapy had proven useful for shrinking some of these adenomas and reducing excess hormone levels. However, none of these cytotoxic agents is curative, and symptoms usually resume shortly after the drug is discontinued. Surgery, particularly through the transsphenoidal approach, is effective and relatively benign, and remains the mainstay of therapy for most pituitary adenomas other than prolactinomas. Radiation therapy has a delayed effect and is complicated by damage to the optic nerves and hypothalamic-pituitary axis as well as by radiation necrosis. Impaired mental status and radiation-induces tumors have also been described. Focused-beam radiation therapy is useful in patients with recurrent or residual tumor, or poor operative candidates whose tumors cannot be controlled with medical therapy. The treatment of specific subtypes of pituitary adenoma is addressed in the following sections.
Prolactinomas
The treatment of prolactinomas remains controversial. Medical therapy with bromocriptine, and ergot derivative, not only shrinks the tumor but also decreases prolactin synthesis and release (49), and is considered first-line therapy for most prolactinomas. This produces relief of symptoms, including galactorrhea and amenorrhea, and can restore fertility. Although accelerated tumor growth can occur during pregnancy, this is rarely significant in microprolactinomas. There have been no teratogenic effects reported from use of bromocriptine to achieve conception. The drug can be discontinued during pregnancy, then re-instituted after delivery. Patients with microprolactinomas desiring fertility can also be treated with transsphenoidal surgery. Surgical cure and subsequent fertility has been reported in 83% of patients (50). Surgery is also indicated for patients with macroadenomas with persistent mass effect despite maximal tolerated dose of bromocriptine; patients who are noncompliant, have serious side effects from bromocriptine, or do not want to continue medical therapy; and women with microprolactinomas desirous of pregnancy. Preoperative prolactin levels correlate both with tumor size and operative success: surgical cure is achieved in 70% of patients with preoperative prolactin levels of 200 to 500 ng/ml, and 14% of patients with prolactin levels over 1000 ng/ml (51).
Bromocriptine is useful for treatment of residual or recurrent prolactinomas in patients who are poor surgical candidates. One caveat is that bromocriptine induces tumor fibrosis, which makes resection difficult and reduces the surgical cure rate by approximately half (52, 53). Radiation therapy is effective at relieving symptoms and reducing prolactin levels. However, because of the effectiveness of surgery and pharmacotherapy for this disorder, radiation therapy is rarely indicated. It is reserved for symptomatic patients with recurrent or residual tumor who are not surgical candidates and are unable or unwilling to control their tumor using bromocriptine.
Growth Hormone-Secreting Adenomas
Excess growth hormone secretion produces not only the visibly obvious deformities of giganticism or acromegaly but also results in severe, systemic metabolic effects that result in a significant decrease in life expectancy. These include atherosclerosis, cardiomyopathy, cerebrovascular disease, respiratory disease, and diseases of the muscles, joints, and peripheral nerves. Thus, the goals for managing growth hormone-secreting adenomas are normalization of growth hormone and somatomedin C levels, in addition to elimination of mass effect.
Two agents have been used for medical therapy of growth hormone-secreting adenomas: bromocriptine and somatostatin. Bromocriptine decreases growth hormone production in 74% of patients, but normalizes levels in only 20% (54). Shrinkage occurs only in isolated cases (55) and requires higher doses of boromocriptine than those used for prolactinomas. These tumors probably represent mixed prolactin cell/growth hormone cell adenomas.
More recently, octreotide, a somatostatin analog, has demonstrated its effectiveness in suppressing growth hormones levels to below 5 mg/ml in 50% of patients, and less than 2 mg/ml in 25% of patients (56). However, this drug must be given by subcutaneous injection two to three times per day, making it suitable only for intelligent, highly motivated patients who can administer the injections themselves. Because of the inconvenience and morbidity of medical treatment, and the effectiveness of surgery in curing most of these patients (57, 58), pharmacologic therapy is reserved for patients who are poor surgical candidates or those with recurrent or residual disease who are unwilling or unable to undergo re-operation. Radiation therapy is reserved for patients in whom an endocrinologic cure cannot be achieved by surgery combined with medical therapy.
Adrenocorticotropic Hormone-Secreting Adenomas
In contrast to hyperprolactinomas, the endocrinopathy of Cushings disease is life threatening. When untreated, the 5-year mortality rate is 50%; thus, the goal of therapy must be endocrinologic cure. Because most corticotropic adenomas are microadenomas, radiologic diagnosis by gadolinium-enhanced MRI is achieved in only 40% to 60% of cases. When a pituitary mass is noted by MRI of the sella, or suggested by diagnostic petrosal sinus sampling in the absence of pulmonary or adrenal lesions on radiologic examination of the thorax and abdomen, transsphenoidal surgery is almost always the treatment of choice. Tumor noted on MRI is usually easily found and resected, while sparing the normal gland. Systematic exploration and sectioning of the gland is indicated to locate radiographically occult adenomas. Usually, several deep vertical and horizontal incisions are made in the gland to find the abnormal tissue. This technique is often successful. When it is not, a total hypophysectomy is usually recommended for older adults, from whom consent should be obtained for this eventuality before surgery. Children and young adults in whom no tumor is found are treated conservatively with medical management and reimaging before further surgery or radiosurgery. However, hypophysectomy is sometimes required by the severity of the endocrinopathy. Resection of the central “mucoid wedge” has also been reported (46, 59). Unilateral hypophysectomy is also an option in patients with occult ACTH-secreting adenomas that have been localized to one side by petrosal sinus sampling (35). Surgical cure has been reported in 90% of microadenomas and 65% of macroadenomas using this technique, with a recurrence rate of 8.5% (60). Successfully treated patients have had an immediate fall in blood cortisol levels with gradual resolution of cushingoid features.
In patients who are refractory to surgical cure, or who are poor operative candidates, pharmacologic treatment or radiation therapy can be used to achieve endocrinologic control. Medical therapy consists primarily of agents that pharmacologically block adrenal steroidogenesis. Ketoconazole is the most useful of these and has been used to stabilize severely ill patients as a preparation for surgery or radiation therapy (61). Other pharmacologic agents such as metyrapone (62), cyproheptadine, a serotonin ingibitor (63), aminoglutethemide, and mitotane (64) have been used to control serum cortisol levels with variable degrees of success.
The delayed effect of radiation therapy makes this modality inadequate as the primary treatment. However, radiation is reported to control disease in 50% to 70% of patients by 3 years (33, 65). This makes it useful as adjuvant treatment when combined with pharmacologic treatment in patients who are poor operative candidates or have persistent or recurrent disease. Bilateral adrenalectomy is immediately effective in curing hypercortisolism, but because of the severe metabolic consequences and the possibility of inducing Nelsons syndrome in patients with occult pituitary adenomas, this procedure is reserved as a last resort in most patients.
Although pituitary adenomas producing Nelsons syndrome are usually macroadenomas and easily visualized, they are characteristically invasive and difficult to cure. Normalization of ACTH and hyperpigmentation occur in less than half the cases (66). Although data is sparse, there is some suggestion that radiation therapy may be somewhat helpful in patients with Nelsons syndrome.
Thyrotropic Adenomas
Thyrotropic adenomas are the least common adenomas, accounting for less than 1% of all pituitary adenomas. They can be microadenomas but often are invasive macroadenomas, particularly in patients who have undergone thyroid ablation. Surgical excision is the treatment of choice and can be achieved in most microadenomas. The surgical cure rate for invasive macroadenomas is only about 40% (67). Octreotide has been reported to reduce TSH levels but does not significantly reduce mass effect, and adjuvant radiation therapy should be used in these cases.
Endocrinologically Inactive Adenomas
Endocrinologically inactive adenomas consist of null cell adenomas and gonadotropin-producing adenomas, which do not produce symptoms of excess endocrine secretion. These collectively account for 25% of all pituitary adenomas. Patients typically present with macroadenomas with mass effect, although they may present with symptoms of mild hyperprolactinemia due to stalk compression. Symptoms of hypopituitarism are often more evident. The goal of surgery is reduction of mass effect, and the treatment of choice is surgery. Although gross total resection is the surgeons intuitive goal, the fact that these tumors occur mainly in the elderly and are slow growing with a high incidence of cavernous sinus invasion suggest that therapy be individualized. The transsphenoidal approach is usually the procedure of choice, and near-total to gross-total resection can usually be achieved, although microscopic invasion is usually present (68). Vision improves or stabilizes in over 90% of these patients (69), and headache and cranial nerve palsies due to cavernous sinus compression often resolve. The patients presenting with panhypopituitarism seldom regain normal pituitary function (70), and they require long-term hormonal therapy. Bromocriptine may decrease prolactin levels through a direct effect on the hypothalamic-pituitary axis. However, given the benign nature of these neoplasms, their slow growth rate, and the success of re-operative surgery, the authors prefer conservative management, or “watchful waiting,” of any residual tumor. If the tumor grows, consideration is given to re-operation before instituting radiation therapy.
Giant Adenomas
The management of giant adenomas is both complex and controversial. These are usually a subset of endocrinologically inactive adenomas presenting with mass effect and obstructive hydrocephalus. Giant adenomas producing prolactin and other hormones have also been reported. Treatment is surgical, but these patients tend to do poorly with a high incidence of morbidity, and a 20% mortality rate (7, 71). The pathophysiology of this phenomenon is unclear, but it is thought to be due to exacerbated hydrocephalus, seemingly related either to tumor swelling after an incomplete resection, or due to a traction effect on the third ventricle. These tumors should be managed conservatively, with debulking through a transsphenoidal approach. Ventricuostomy can be done before or during surgery if hydroephalus is present. Complete resection is rarely required or achieved. Regional radiation therapy may be useful in poor operative candidates.
Apoplexy
Pituitary apoplexy is a clinical emergency requiring urgent surgical decompression of the sella and parasellar structures, usually through a transsphenoidal approach.
Pituitary Carcinoma
Pituitary carcinoma is rare, with less than 40 cases reported (72). They appear to arise from pituitary adenomas of all types, although endocrinologically active adenomas permeate. They typically present with multiple local recurces followed by metastatic dissemination to the subchnoid space or extraneuronal sites such as bone, liver, limp node, lung, or kidney. This is usually accompanied by escalation of histologic grade. The most symptomatic re is usually the sella, and the therapy is directed at control the primary neoplasm. Aggressive re-operation is used when adjuvant radiation therapy and pharmacologic therapy indicated by the characteristics of the original adenoma. In most cases, death from pituitary carcinoma results from a failure of local control with progressive local invasion, rather than from metastatic disease.
Tumors of the Posterior Pituitary
Granular cell tumors, or choristomas, are usually benign and asymptomatic, although malignant transformation has been reported. Symptomatic granular cell tumors most often present with visual disturbance, although hypopituitarism, hyperprolactinemia, precocious puberty, and acromegaly also occur. Diabetes insipidus is rare. Transsphenoidal surgery is usually indicated for diagnosis and resection. There is no evidence that these tumors are radiosensitive (13).
Management of metastatic lesions is more complex. Although most metastases to the neurohypophysis are asymptomatic, metastases to the stalk may present with hypopituitarism or mass effect (13). Treatment is palliative, and consists of surgery, radiation therapy, chemotherapy, or combinations of these modalities depending on the need for tissue confirmation, tumor type, and the medical condition of the patient. Usually, metastases are amenable to excision using the transsphenoidal approach if this is clinically indicated, and surgery can prolong survival.
Craniopharyngiomas
Craniopharyngiomas are among the most challenging tumors to treat. Although they are slow growing, benign, extraaxial neoplasms, some consider them malignant by virtue of location. Their tenacious attachment to pial vessels and critical neurovascular structures increases the morbidity of aggressive resection. Yet, the high recurrence rate and consequent morbidity after subtotal resection make this option equally distasteful.
The presenting symptoms depend on the size and location of the tumor. Craniopharyngiomas present with mass effect and symptoms of increased intracranial pressure in 80 % of adults. Endocrine dysfunction, including partial or complete hypopituitarism and diabetes insipidus, occurs in most patients of all ages, although it is usually not the presenting symptom.
Craniopharyngiomas are classified as endosellar, suprasellar, or intraventricular based on their position relative to the sella, chiasm, and third ventricle (73). Endosellar craniopharyngiomas are subdiaphragmatic and confined to the sella. They comprise 3% to 8% of craniopharyngiomas (74). Suprasellar craniopharyngiomas arise from the mid-portion of the stalk and typically enlarge beneath the third ventricle, elevating the third ventricular floor. Intraventricular craniopharyngiomas arise from the infundibulum. They invade the floor of the third ventricle and often extend into the interpeduncular fossa. The latter two types are considered retrochiasmatic craniopharyngiomas.
There is general agreement that surgery is the treatment of choice for craniopharyngiomas. However, the choice of approaches, the extent of resection, and the use of adjuvant radiation therapy remain controversial. The difficulty is that these tumors recur unless gross total resection is achieved, and many believe that the initial operation represents the only opportunity for cure. However, the tumor capsule is tenaciously attached to vital structures such as the optic chiasm, optic tract, infundibulum, pituitary, hypothalamus, third ventricle, and the vessels that supply these critical structures. Despite advances in microsurgical technique, aggressive resection of these densely adherent tumors has resulted in a high rate of neurovascular and endocrine complications. Even in experienced hands, the mortality rate for primary craniopharyngiomas has been reported to be as high as 8% to 15%, with varying degrees of visual compromise in 50% to 60% of patients, and 80% of patients require postoperative endocrine replacement (75, 76). This morbidity is thought to result from devascularization of critical structures due to aggressive resection of tumor capsule. Thus, it is preferable to leave densely adherent capsule attached to critical neurovascular structures rather than pursue more aggressive resection of tumor capsule. This, it is preferable to leave densely adherent capsule attached to critical neurovascular structures rather than pursue more aggressive resection that may lead to unacceptable morbidity.
The choice of surgical approach depends on the location, shape, and size of the tumor. Endosellar lesions with an enlarged sella and a prominent cystic component can be safely approached through the transsphenoidal route. Because of their subdiaphragmatic position, these tumors are not attached to the hypothalamus or optic chiasm. After draining the cystic component under direct vision, the intracapsular contents are removed with a grasping forceps and curettes. The capsule collapses and can be detached from the under surface of the diaphragma sellae. Normal preoperative pituitary function is a relative contraindication to the transsphenoidal approach because the gland is often situated anteriorly within the sella and damaged during tumor resection. The stalk can usually be preserved.
Suprasellar craniopharyngiomas are best resected using transcranial approaches. If hydrocephalus is present, ventricular drainage should be instituted before definitive therapy. The most useful approaches are the pterional and the subfrontal. Both have the advantage of allowing direct visualization of the optic nerve and chiasm. The pterional approach is the shortest and offers superior visualization of the retrochiasmatic region. The subfrontal approach provides better visualization of the optic pathways by way of the subchiasmatic pathway, as well as a midline route to the lamina terminalis. The opticocarotid pathway may also be useful. In addition, access to the sella can be achieved by removing the tuberculum sellae with a high-speed drill.
Intraventricular craniopharyngiomas are best approached by the subfrontal-lamina terminalis approach, the interhemispheric-transcallosal approach, the interhemispheric-transcallosal approach, or a combination of these. The subfrontal-lamina terminalis approach allows direct visualization of the chiasm and permits good access to the anterior third ventricle. However, this approach also carries the greatest risk of hypothalamic damage. The interhemispheric-transcallosal approach utilizes the foramina of Monro, which are usually enlarged due to hydrocephalus, to resect the intraventricular portion of the tumor. Alternately, if the foramina of Monro is not enlarged, the interforniceal approach may be used. Although the latter two approaches minimize the risk of devascularizing the hypothalamus, they carry the risk of bilateral forniceal damage and consequent memory impairment. Because these approaches give access only to intraventricular tumor, they are often combined with pterional or subfrontal approaches. The transcortical approach is associated with a high risk of hydrocephalus and postoperative seizures, in addition to the aforementioned risk of bilateral forniceal injury, and should be avoided except in cases of marked preoperative ventricular dilatation.
In all approaches, the presence of a subarachnoid plane facilitates complete resection and preservation of the normal vasculature. Electrocautery often disrupts this interface and should be avoided whenever possible. Although radiation therapy has been effective in reducing recurrence and increasing survival in some series with minimal morbidity (77), the risks of radiation therapy have prompted the authors to follow patients with serial examination and imaging studies, and individualize treatment of recurrence.
Meningiomas
Usually, parasellar meningiomas are large, and present predominantly with mass effect. Visual loss is the predominant symptom. Pituitary insufficiency is uncommon and usually mild. Because resection of parasellar meningiomas requires resection of the dura itself, an intradural approach is required for complete resection. The choice of approach I governed by the location and orientation of the lesion. Meningiomas of the tuberculum sellae, olfactory groove, and anterior clinoids are best approached through a subfrontal approach. Meningiomas of the medial sphenoid wing and cavernous sinus require a pterional approach, whereas retrochiasmal meningiomas are usually best resected using the subtemporal approach. The transsphenoidal approach may be indicated for diagnostic biopsy.
Chordomas
Chordomas of the sella typically present with symptoms of mass effect and hypopituitarism depending on their size and direction of growth. Occasional small tumors confined to the sella can be resected through the transsphenoidal approach alone. At the end of such an operation, the clivus should be drilled beyond the obvious tumor margin because of invasion within cancellous lumen. A tumor-free margin of well vascularized bone should be observed. The clival dura should be exposed and prolapse freely into the bony defect. However, most chordomas involve parasellar structures as well. Because gross total resection is associated with increased survival (16), such patients require a more extensive extradural or transcranial approach than can be achieved by the transsphenoidal approach alone. An extended transsphenoidal, transmaxillary, or transoral approach may be required to maximize exposure in these cases. There are some studies suggesting that adjuvant proton-beam radiation therapy may also prolong survival (78).
Nonneoplastic Cysts
Rathkes cleft cysts, mucoceles, and arachnoid cysts are the most common nonneoplastic cysts of the sella. They are usually asymptomatic. Occasionally, however, patients do present with symptoms of mass effect, hypopituitarism, or mild hyperprolactinemia due to stalk compression. Suprasellar extension may occasionally produce visual loss or hypothalamic dysfunction. Transsphenoidal decompression of the cyst, or marsupialization, without excision of the cyst wall is usually curative.
Inflammatory and Infectious Lesions
Inflammatory Lesions
Lymphocytic hypophysitis, Langerhans cell histiocytosis, giant cell granuloma, and sarcidosis are the most common inflammatory lesions of the sella turcica. These sellar lesions are often indistinguishable from pituitary adenoma on MRI. Transsphenoidal biopsy is required for diagnosis, but radical resection is rarely indicated. Lymphocytic hypophysitis typically presents with mass effect, symptoms of moderate hyperprolactinemia, and varying degrees of hypopituitarism, although diabetes insipidus is unusual. At surgery, the adenohypophysis is yellow, rubbery, and firm, and the inflammatory mass frequently extends into the suprasellar region. Patients are started on hormonal replacement ant treated with steroids.
Langerhans cell histiocytosis (histiocytosis X) of the sella usually presents with diabetes insipidus because of its preferential involvement of the stalk and posterior pituitary gland. Mild to moderate hypopituitarism may also be present. These lesions should be resected. Adjuvant radiation therapy has also been used (13).
Granulomatous lesions such as giant cell granuloma and sarcoidosis present with hypopituitarism and, occasionally, hyperprolactinemia. Sarcoidosis may also produce mass effect, diabetes insipidus, and even hypothalamic dysfunction. After biopsy to exclude infectious processes, they are treated conservatively with endocrine replacement, if required, and steroids.
Infectious Lesions
Abscesses of the pituitary typically present with symptoms of mass effect and moderate to severe hypopituitarism. In the setting of meningitis, this diagnosis should always be considered. The transsphenoidal approach is used to debulk and to obtain a diagnostic specimen for aerobic, anaerobic, acid-fat, and fungal cultures. Patients are then treated with the appropriate antibiotic, antituberculous, or antifungal agents.
Vascular Lesions
Aneurysms and carotid cavernous fistulas of the infraclinoid or cavernous carotid may present with a clinical picture indistinguishable from that of an endocrinologically inactive pituitary adenoma. Visual deficit, mild hyperprolactinemia, and hypopituitarism are common. Fortunately, the diagnosis is readily made by MRI, magnetic resonance angiography, or conventional angiography. The treatment of aneurysms and carotid-cavernous fistulas is beyond the scope of this article.
REFERENCES
- Renn WH, Rhoton Al Jr. Microsurgical anatomy of the sellar region. J Neurosurg 1975;43:288-298.
- Tindall GT, Barrow DL,eds. Disorders of the pituitary. St. Louis: Mosby, 1986.
- Lang R. Anatomy of the sellar region. In: Samii M. Draf W, eds. Surgery of the skull base: an interdisciplinary approach. Berlin: Springer-Verlag, 1989:27-35.
- Annegers JF, Coulam CB, Abboud CF, et al. Pituitary adenomas in Olmsted County, Minnesota, 1935-1977. Mayo Clin Proc 1978:53:641-643.
- Costello RT. Subclinical adenoma of the pituitary gland. Am J Pathol 1936;12:205-216.
- Hardy J. Transsphenoidal microsurgery of the normal and pathologic pituitary. Clin Neurosurg 1969;16:185-217.
- Symon L. Jakubowski J, Kendall B. Surgical treatment of giant pituitary adenomas. J Neurol Neurosurg Psychiatry 1979;42:973-982.
- Wilson CB. Neurosurgical management of large and invasive pituitary tumors. In: Tindall GT, Collins R, eds. Clinical management of pituitary disorders. New York: Raven Press, 1979:334-342.
- Ridgeway EC, Klibanski A, Ladenson PW, et al. Pure alpha-secreting adenomas. N Engl J Med 1981;304:1254-1259.
- Kovacs K, Horvath E. Tumors of the pituitary gland. In: Atlas of tumor pathology. 2nd series, fascicle 21. Washington, DC: Armed Forces Institute of Pathology, 1986.
- Scheithauer BW, Kovacs K, Laws ER Jr. The pathology of invasive pituitary tumors with special reference to their functional classification. J Neurosurg 1986;65:733-744.
- Luse SA, Kernohan JW. Granular cell tumors of the stalk and posterior lobe of the pituitary gland. Cancer 1955;8:616-622.
- Albrecht S, Bilbao JM, Kovacs K. Nonpituitary tumors of the sellar region. In: Melmed S, ed. The pituitary. New York: Blackwell Science, 1995:591-676.
- Canbus B, Akar N, Ciplak G, et al. Tumors of the sellar-parasellar region: analysis of 238 cases. In: Samii M, ed. Skull base surgery. Basel: Karger, 1992:317-320.
- Grisoli F, Vincentelli F, Raybaud C, et al. Intrasellar meningiomas. Surg Neurol 1983;20:36-41.
- Gay E, Sekhar LN, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neuro-surgery 1995;56:887-897.
- Mathews W, Wilson CB. Ectopic intrasellar chordoma. J Neurosurg 1974;40:260-263.
- Max MB, Deck MDF, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology 1981;31:998-1002.
- Roessmann U, Kaufmann B, Friede RL. Metastatic lesions in the sella turcica and pituitary gland. Cancer 1970;25:478-480.
- Branch CL. Laws ER. Metastatic tumors of the sella trucica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 1987;65:469-474.
- Buchmann E. Schweisinger G. Hypophyse and Hamoblastosen. Zentralbl Neurochir 1979;40:35-40.
- Shanklin WM. On the presence of cysts in the human pituitary. Anat Rec 1949;104:379-407.
- Lee JH. Laws ER Jr, Guthrie BL. Et al. Lymphocytic hypophysitis: occurrence in two men. Neurosurgery 1994;34:159-163.
- Nishio S. Mizuno J. Barrow DL. Et al. Isolated histiocytosis X of the pituitary gland. Neurosurgery 1987;21:718-721.
- Taylon C, Duff TA. Giant cell granuloma involving the pituitary gland. J Neurosurg 1980;52:584-587.
- Stern BJ. Krumholtz A, Johns C. et al. Sarcoidosis and its neurological manifestations. Arch Neurol 1985;42:909-917.
- Berger SA, Edberg SC, David G. Infectious disease in the sella turcica. Rev Infect Dis 1986;8:747-755.
- Domingue JN, Wilson CB. Pituitary abscesses: report of seven cases and review of the literature. J Neurosurg 1977;46:601-608.
- Ramos-Gabatin A, Jordan RM. Rimary pituitary aspergillosis responding to transphenoidal surgery and combined therapy with amphotericin-B and 5-fluoro-cytosine: case report. J Neurosurg 1981;54:839-841.
- Del Brutto OH. Guevar J, Sotelo J. Intrasellar cysticercosis. J Neurosurg 1988;69:58-60.
- Osgen T, Bertan V, Kansu T. et al. Intrasellar hydatid dyst: case report. Neurosurgery 1984;60:647-648.
- Martin JB. Management of hypersecretory pituitary adenomas. Clin Neurosurg 1980;27:99-247.
- Orth DN. Liddle CW. Results of treatment in 108 patients with Cushings syndrome. N Engl J Med 1971;285:243.
- Wilson CB, Role of surgery in the management of pituitary tumors. Neurosurg Clin North Am 1990;1:139-159.
- Benoit R. Pearson-Murphy BE, Robert F. et al. Hyperthyroidism due to a pituitary TSH secreting tumor with amenorrhea-galactorrhea. Clin Endocrinol 1980:12:11-14.
- Melmed S. ed. The pituitary. New York: Blackwell Science. 1995.
- Ciric I. Mikhael M. Stafford T, Lawson L, Garces R. Transsphenoidal management of pituitary macroadenomas with long term follow-up results. J Neurosurg 1982; 59:395-401.
- Cohen AR, Cooper PR, Kupersmith MJ, et al. Visual recovery after transsphenoidal removal of pituitary adenomas. Neurosurgery 1985; 17:446-152.
- Nicola G. Transsphenoidal surgery for pituitary tumors with extrasellar extension. Progress in Neurological Surgery 1975;6:149-164.
- Symon L. Jakubowski J. Transcranial management of pituitary tumors with extrasellar extention. J Neurol Neurosurg Psychiatry 1979;42:123.
- Schloffer H. Erfulgreiche Operation eines Hypophysentumors suf Nasalm Wege. Wien Klin Wochamschar 1907;20:621.
- Weiss MH. Transnasal transsphenoidal approach. In: Apuzzo MJ, ed. Surgery of the third ventricle. Baltimore: Williams & Wilkins, 1987:476-494.
- Hardy J. Transsphenoidal approach to the sella. In: Wilson CD. Ed. Neurosurgical procedures. Baltimore: Williams & Wilkins, 1992:21-40.
- King WA, Becker DP. Transphenoidal resection of cavernous sinus tumors. In: Samii M. ed. Skull base surgery, Base: Karger, 1992:458-463.
- Chiari O. Uever eine Modification der Schlofferschen Operation von Tumoren der Hypophyse. Wien Klin Wochenschr 1912;25:5-6.
- Black PM, Zerevas NT. Surgical management of sellar and parasellar lesions. In: Schmidik R, Sweet T, eds. Operative neurosurgical techniques. 2nd ed. New York: Grune & Stratton. 1988:300-307.
- Lalwani AK, Kaplan MJ, Gutin PH. The transphenoethmoid approach to the sphenoid sinus and clivus. Neurosurgery 1992;31:1008-1014.
- Galfinos JG, Fitzpatrick BC, Smith LR, et al. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg 1995;83:197-205.
- Landolt AM. Prolactinomas: preoperative bromocriptine treatment. Perspectives in Neurologic Surgery 1990;1:105-115.
- Laws ER Jr. Fode NC, Randal RV, et al. Pregnancy following transsphenoidal resection of prolactin-secreting pituitary tumors. J Neurosurg 1983;58:685-688.
- Tindall GT. McLanahan CS. Christy JH. Transphenoidal microsurgery for pituitary tumors associated with hyperprolactinemia. J Neurosurg 1978;48:849-860.
- Landolt AM, Keller PJ, Froesch ER, et al. Bromocriptine: does it jeopardize the result of later surgery for prolactinomas? Lancet 1982;2:657-652.
- Landolt AM. Osterwalder V. Perivascular fibrosis in prolactinomas: is it increased by bromocriptine? J Clin Endocrinol Metab 1984;58:1179-1182.
- Besser GM, Wass JAH, Thorner MO. Bromocriptine in the medical management of acromegaly. Adv Biochem Psychopharmacol 1980;23:191-198.
- Oppizzi C, Luizzi A, Chiodini P, et al. Dopaminergic treatment of acromegaly: different effects of hormone secretion and tumor size. J Clin Endocrinol Metab 1984;58:988-992.
- Ezzat S, Snyder PJ, Young WF, et al. Octreotide treatment of acromegaly: a randomized multicenter study. Ann Intern Med 1992;117:711-718.
- Laws ER JR, Piepgras DG, Randall RV. Neurosurgical management of acromegaly: results in 82 patients treated between 1972 and 1977. J Neurosurg 1979;50:454-461.
- Laws ER Jr, Rnadall RV, Abboud CF. Surgical treatment of acromegaly: results in 140 patients. In: Givens J, ed. Hormone secreting pituitary tumors. Chicago: Year Book, 1982:225-228.
- Hardy J. Atlas of transsphenoidal microsurgery in pituitary tumors. New York: Igaku-Shoin, 1991.
- Salassa RM, Laws ER Jr, Carpenter RC, et al. Transsphenoidal removal of pituitary microadenoma in Cushings disease. Mayo Clin Proc 1978;53:24-28.
- Sonino N, Boscaro M, Merola G, et al. Prolonged treatment of Cushings disease by ketoconazole. J Clin Endocrinol Metab 1985;61:718-722.
- Jeffcoate WJ, Rees LH, Tomlin S, et al. Metyrapone in long-term management of Cushings disease. Br Med J 1977;2:215-217.
- Krieger DT, Amorosa L, Linick F. Cyproheptadine-induced remission of Cushings disease. N Engl J Med 1975;293:893-896.
- Luton JP, Mahoudeau JA, Bouchard P, et al. Treatment of Cushings disease by o,p-DDD: survery of 62 cases. N Engl J Med 1979;300:459-464.
- Edmonds MW, Simpson WJK, Meakin JW. External irradiation of the hypophysis for Cushings disease. Can Med Assoc J 1972;107:860-862.
- Thapar K, Smith M, Elliott E, et al. Corticotroph adenomas of the pituitary: long term results of operative treatment. Endocrine Pathology 1992;31:553-555.
- Thapar K, Laws ER Jr. Pituitary tumors. In: Laws ER Jr, ed. Brain tumors: basis for individual management. Philadelphia: JB Lippincott, 1995:759-773.
- Selman WR, Laws ER Jr, Scheithauer BW, et al. The occurrence of dural invasion in pituitary adenomas. J Neurosurg 1986;64:402-407.
- Trautmann JC, Laws ER JR. Visual status after transsphenoidal surgery at the Mayo Clinic, 1971-1982. Am J Ophthalmol 1983;96:200-208.
- Nelson AT JR, Tucker H St G. Becker DP. Residual anterior pituitary function following transsphenoidal resection of pituitary macroadenoma. J Neurosurg 1984;61:577-580.
- Decker RE, Chalif DJ. Progressive coma after the transphenoidal decompression of a pituitary adenoma with marked suprasellar extension. Neurosurgery 1991;28:154-158.
- Pernicone PJ, Scheithauer BW. Invasive pituitary adenomas and pituitary carcinomas. In: Lloyd RV.ed. Surgical pathology of the pituitary gland. Philadelphia: WB Saunders, 1993:121-136.
- Northfield DWC, Rathke-pouch tumors. Brain 1957;80:293-301.
- Litofsky NS, Levy ML, Apuzzo MLJ. Craniopharyngioma. In: Apuzzo MLJ, ed. Brain surgery: complication avoidance and management. New York: Churchill Livingstone, 1993:313-378.
- Yasargil MG, Curcic M, Kis M, et al. Total removal of craniopharyngiomas: approaches and long-term results in 144 patients. J Neurosurg 1990;73:3-11.
- Baskin DS, Wilson CP. Surgical management of craniopharyngiomas: a review of 74 cases. J Neurosurg 1986;65:22-27.
- Hug EB, Fitzek MM, Liebsch HJ, Muzenrider KJE. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys 1995;31:467-476.
- Austin-Seymour M. Munzenrider J, Goitein M, et al. Fractionated proton radiation therapy of chordoma and lowgrade chordosarcoma of the base of the skull. J Neurosurg 1989;70:13-17.
Transsphenoidal Surgery: How Its Done, What To Expect
Donald P. Becker, M.D.
Indications
 Figure 1: Patient position for transsphenoidal approach.
Figure 1: Patient position for transsphenoidal approach.
The transsphenoidal approach is the procedure of choice for the removal of tumors of the sella that are still largely confined to this space. It is also indicated for removal of some tumors of the parasellar area. Pituitary tumors with substantial intracranial extension can engulf important structures and should be treated using a transcranial approach.
For transsphenoidal surgery, there are several alternative approaches to the sphenoid sinus. These include the transnasal paraseptal, sublabial-transseptal, and paranasal transethmoidal approaches. For processes that are largely located in the sella, the sublabial or trananasal approach is used. With extreme involvement of the paranasal sinuses, the paranasal transethmoidal exposure is of benefit.
Position
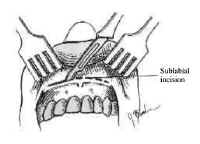 Figure 2: Sublabial incision.Proper positioning is essential in transphenoidal surgery to ensure adequate expsure of the sphenoid sinus structures, to minimize bleeding, and to maximize the surgeons comfort It is also crucial in helping to ensure that the operative dissection remains midline.
Figure 2: Sublabial incision.Proper positioning is essential in transphenoidal surgery to ensure adequate expsure of the sphenoid sinus structures, to minimize bleeding, and to maximize the surgeons comfort It is also crucial in helping to ensure that the operative dissection remains midline.
The patient is positioned supine with the head elevated to 30 degrees, turned 20 degrees toward the surgeon, and flexed toward the opposite shoulder (Figure 1).
The head is placed in a head immobilizer or, alternatively, supported with a padded ring. We prefer the latter because this allows subtle movement of the operative field intraoperatively without the need for microscope repositioning. The nurse stands next to the surgeon, and the patient is positioned at the head of the table (see Figure 1).
Operative Procedure
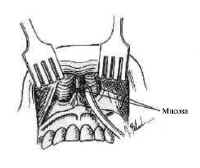 Figure 3: Elevation of the inferior mucosa within each aperture.We describe in this chapter the sublabial approach for transsphenoidal surgery.
Figure 3: Elevation of the inferior mucosa within each aperture.We describe in this chapter the sublabial approach for transsphenoidal surgery.
A sublabial incision positioned on the gingival mucosa well above the teeth is made extending between the canine teeth (Figure 2).
Periosteal elevators are used to elevate the mucosa off the maxilla. After the pyriform apertures are identified and defined, the inferior mucosa within each aperture is elevated along the premaxilla, forming inferior tunnels (Figure 3).
The cartilaginous septum is exposed using sharp dissection, and a submucosal tunnel is -elevated along its left side. Fibrous adhesions at the base of the cartilage must be divided sharply, allowing the septum to be disarticulated from the nasal spine and maxilhry ridge. The cartilage can then be hinged to the patients right side. Increased exposure may require enlargement of the pyrlibrm apertures with rongeurs or removal of the nasal spine (Figure 4).
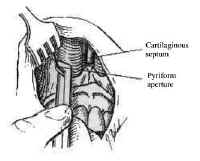 Figure 4: Enlargement of the pyriform apertures.Posteriorly, the mucosa is elevated from both sides of the bony septum, which subse quendy is fractured, removed, and saved. Under microscopic magnification, the characteristic keel appearance of the vomer is seen during this phase (Figure 5).
Figure 4: Enlargement of the pyriform apertures.Posteriorly, the mucosa is elevated from both sides of the bony septum, which subse quendy is fractured, removed, and saved. Under microscopic magnification, the characteristic keel appearance of the vomer is seen during this phase (Figure 5).
The anterior wall of the sphenoid sinus is confirmed by identifying the ostea, located superolaterally, or with a lateral radiograph. The sinus is opened widely, and all mucosa is removed from within it to prevent trouble some bleeding. Placement of the speculum within the sphenoid sinus should be avoided because fracture of the sphenoid bone can result in injury to the optic nerve, cranial nerves, and carotid artery. After the mucosa is removed, the bulge of the sella is readily apparent and, typically, is thinned by the tumor. If necessary, the- bone of the sella can be fractured with a small osteotome or removed wid1 a diamond drill, exposing the medial extent of each cavernous sinus (Figure 6). In rare cases, the sphenoid sinus is incompletely pneumatized, requiring removal of bone with a high speed drill to expose the sella.
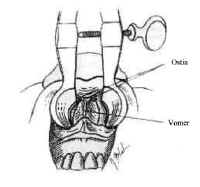 Figure 5: Visualization of the characteristic keel of the sphenoid sinus.The dura is incised in a cruciate manner after coagulating it with the bipolar electrocautery. Intercavernous venous channels that extend across the sella frequently result in brisk bleeding when the dura is incised The bleeding can be controlled by grasping both, leaves of the dura with the bipolar forceps and coagulating.
Figure 5: Visualization of the characteristic keel of the sphenoid sinus.The dura is incised in a cruciate manner after coagulating it with the bipolar electrocautery. Intercavernous venous channels that extend across the sella frequently result in brisk bleeding when the dura is incised The bleeding can be controlled by grasping both, leaves of the dura with the bipolar forceps and coagulating.
Pituitary adenomas usually are soft and can be readily removed with bayonetted ring curettes. Intermittent. Valsalva maneuvers by the anesthesiologist expedite tumor removal by elevating the subarachnoid fluid pressure, which is transmitted to the tumor capsule, displacing tumor tissue downward.
After the bulk of the tumor is removed, the sella is inspected systematically and the pituitary gland itself is examined. If the tumor capsule prolapses into the sphenoid sinus or cerebrospinal fluid leakage occurs, fascia lata or adipose tissue and fibrin glue may be used to occlude the communication to the subarachnoid space and support the diaphragma sellae. The floor of the sella can be reconstructed vvith the bony septum previously removed. After complete hemostasis is achieved, the speculum is removed and the sublabial mucosa is closed with interrupted absorbable structures.
 Figure 6: Removal of the floor of the sphenoid sinus.Petroleum jelly-covered gauze is packed into each nasal passage and left in place for 48 to 72 hours.
Figure 6: Removal of the floor of the sphenoid sinus.Petroleum jelly-covered gauze is packed into each nasal passage and left in place for 48 to 72 hours.
In the case of extensive dural defect, lumbar cerebrospinal fluid drainage for 2 to 3 days is advisable.
Cavernous Sinus Extension of Tumors
Some tumors extend in the lateral direction, requiring surgicalentry into the medial cavernous sinus. In these cases, the surgeonmust be familiar with the important anatomical structures adjacentto the sella before proceeding with surgery. The structures mostat risk include the internal carotid artery and its intracavernousbranches as well as cranial nerves to extraocular muscles.
Sometimes better exposure of the medial wall of the sphenoidsinus and cavernous sinus is necessary in these situations Thiscan be gained by enlarging the pyriform aperture or by removingthe medial maxillary sinus wall contralateral to the involvedcavernous sinus and directing the speculum laterally.
Tumors that involve the medial cavernous sinus typicallyobliterate the venous channels and can be removed piecemeal usingmicrodissectors. These instruments can be gently used to removethe tumor around the carotid artery with special care to avoidinjury to the ocular muscle nerves, particularly the abducensnerve.
Significant venous bleeding is not a problem until the finalstages of tumor removal as the patent part of the sinus isexposed. As with other approaches to the cavernous sinus, thetumor should be removed in an organized manner and bleedingcontrolled with carefully placed oxidized cellulose and pressure.
Recommended Reading
Balagura, S., Tindall, G.: Technique of transsphenoidalMicrosurgery. Contemp. Neurosurg. 3(24): 1982.
Eisele, D., Flint, P., Janas, J., et al.: The sublabialtransseptal transsphenoidal approach to sellar and parasellarlesions. Laryngoscope 98:1301-1308,1988.
Tindall, G., Herring, C., Clark, R., et al.: Cushingsdisease: Results of transsphenoidal microsurgery with emphasis onsurgical failures. J. Neurosurgery. 72:363-369, 1990.
Tucker, H., Hahn, J.: Transnasal, transseptal sephenoidalapproach to hypophysectomy. Laryngoscope 92:55-57, 1982
Welbourn, R.: The evolution of transsphenoidal pituitarymicrosurgery. Surgery 100(6):1185-7789, 1986
Pituitary Diseases During Pregnancy
Martin N. Montoro, MD, and Jorge H. Mestman, MD
Changes In The Normal Anterior Pituitary Gland During Pregnancy
Anatomic and functional changes take place in the pituitary gland during pregnancy that have to be taken into consideration to properly evaluate its function, and particularly to diagnose pituitary disorders during pregnancy.
The various anterior pituitary hormonesprolactin (PRL), thyroxine-stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH), corticotropin (ACTH), and growth hormone (GH) are released into the systemic circulation in response to stimulating or inhibiting messages from hypothalamic peptide hormones. These peptides reach the pituitary gland through the portal circulation. Thus far several have been identified; they include thyrotropin-releasing hormone (TRH), gonadotropin-releasing hormone GnRH), corticotropin-releasing factor (CRF), growth hormone-releasing factor (GHRF), somatostatin (somatotropin release-inhibiting factor [SRIF]) and more recently, a GnRH-associated peptide (GAP) that inhibits prolactin secretion. Dopamine, a biogenic amine, also inhibits PRL secretion. Communications between the hypothalamus and the higher centers of the brain are accomplished by a variety of neurotransmitters, which include the already mentioned dopamine, serotonin, y-aminobutiric acid (GABA), norepinephrine and various opioid peptides, as well as many of the peptides also found in the intestinal tract.
The volume of the anterior pituitary increases progressively during pregnancy and gradually decreases after delivery over a period of several months; the postpartum involution is slower in lactating women. The enlargement is more pronounced in multiparous than in primiparous women. The pituitary volume may increase by one third or more at term, and it is due to both hyperplasia and hypertrophy of the PRL-secreting cells. The pituitary volume, as determined by magnetic resonance imaging (MRI) or at autopsy, has been measured at 300+/- 60 mm in non-pregnant controls and 437 +/- 90 mm, 534 +/- 124 mm, and 702 +/- 123 mm in the first, second, and third trimesters, respectively. The PRL levels increase gradually from early pregnancy up to tenfold above the non-pregnant levels by term (mean, 50 ng/ml at 12 weeks to 270 ng/ml at term; range for the rapid, transient elevations associated with breast-feeding. These gradual changes are thought to be secondary to the stimulating effect of estrogen and perhaps, progesterone. Despite the great PRL elevation, normal diumal rhythm and response to TRH are maintained, as opposed to the hyperprolactinemia of the various disease states (e.g., tumors). The role of PRL elevation during pregnancy remains unclear, but it is believed to be related to the initiation and maintenance of lactation. Some investigators have suggested that PRL plays a role in some of the metabolic changes that occur in pregnancy and perhaps in fetal lung maturation, but the evidence remains inconclusive.
The levels of LH and FSH decrease to the point of becoming undetectable, and the response to GnRH becomes blunted or absent. These changes are thought to be the result of feedback inhibition from the elevated levels of estrogen and progesterone.
Basal GH levels remain with little change, but the response to the usual stimulating tests (hypoglycemia, L-arginine, etc.) becomes blunted and remains so until several weeks after delivery. Various factors have been implicated; until recently the most important was attributed to the elevated levels of human placental lactogen (hPL) to which GH-like activity is attributed. With the advent of more specific assays capable of overcoming interference with hPL, however, the hypersomatotrophism of pregnancy now is attributed to a specific placental GH.
Maternal pituitary TSH may be suppressed during the first several weeks of gestation, and for this reason it may not be used as effectively as a diagnostic test during the early part of gestation (about the first trimester). It is believed that TSH suppression occurs when the levels of human chorionic gonadotropin (hCG) are highest, because hCG has TSH-like activity.
The ACTH levels increase gradually while pregnancy progresses, despite the also gradually increasing levels of both free and bound cortisol seen during pregnancy (because of increased cortisol binding globulin, increased cortisol production rate, and perhaps reduced clearance). Also, an exaggerated cortisol response to ACTH occurs, even in the presence of elevated basal cortisol levels. A CRF has been found in the placenta; the levels of this factor increase gradually with progression of pregnancy, peaking at term and falling abruptly after the delivery of the placenta. It remains unknown whether CRF could be the cause of some of the cortisol changes during pregnancy or whether it may play a role in certain cases of Cushings syndrome. Suppression to exogenous corticosteroid administration is incomplete, but the diumal variations of both ACTH and cortisol are preserved, thus making the measurement of morning and evening cortisol levels a useful test. Free urinary cortisol excretion increases to levels that may overlap with those seen in some cases of Cushings syndrome.
Disorders of the Anterior Pituitary Gland
Anterior Pituitary Insufficiency
The most common causes of partial or complete anterior pituitary insufficiency in women of childbearing age are tumors, hypophysectomy, radiation therapy, and postpartum infarction (Sheehans syndrome). Less common causes include acute intrapituitary hemorrhage, infiltration by granulomatous diseases, some hemoglobinopathies (e.g., thalassemia), and pituitary necrosis in the presence of elevated intracranial pressure. More recently, lymphocytic hypophysitis has come to be recognized as a distinct clinical entity that may present as partial or total anterior pituitary insufficiency during pregnancy or the postpartum period. A few cases of acute pituitary insufficiency during pregnancy in insulin dependent diabetics also have been described.
Pituitary insufficiency occurring for the first time during pregnancy is uncommon, but may occur because of enlargement or infarction of a pituitary tumor, in lymphocytic hypophysitis, and rarely in diabetes mellitus. It may occur as an acute event, which is what usually happens when pituitary apoplexy develops because of pituitary infarction; other times, in tumors or in lymphocytic hypophysitis, compression symptoms due to mass effect or only partial pituitary insufficiency may occur first. In these cases there exists the possibility of making the diagnosis and initiating treatment before acute pituitary insufficiency occurs as a catastrophic event, which may result in maternal or fetal death or both.
Given the recent advances in the treatment of infertility, it is now possible for women with anterior pituitary insufficiency not only to conceive, but also to carry a pregnancy to term with adequate hormonal replacement. Table 1 shows the commonly used tests to assess pituitary reserve and the results in normal pregnancy.
Sheehans Syndrome
Sheehans syndrome is defined as partial or complete hypopituitarism due to pituitary necrosis after deliveries in which severe blood loss and hypotension have occurred. In about 10% of Sheehans syndrome cases, however, no history of bleeding or hypotension can be documented. Clinically there is failure to lactate, amenorrhea, loss of pubic and axillary hair, or failure of pubic hair to grow back if it was shaved (e.g., after a cesarean section). The skin becomes waxy and the patients develop fine skin wrinkles around the eyes and the corners of the mouth. In more severe cases (when 90% or more of the pituitary is destroyed) anorexia, nausea, vomiting, hypoglycemia, and hypotension may develop (signs of adrenal insufficiency). Other symptoms include postural hypotension, weight loss, fatigue, loss of libido, and breast atrophy. Abnormal laboratory tests may include normochromic, normocytic anemia, and hyponatremia. It is typical, however, for the full-blown picture to take many years (even 10 to 20) to develop with less extensive pituitary destruction. Even in advanced cases of hypopituitarism, enough adrenal reserve still may be present to cope with normal everyday activities, and the diagnosis may be made for the first time when acute adrenal insufficiency develops after a stressful situation such as surgery, infection, or any other serious illness. To exclude a tumor or other pathologic abnormality, the diagnosis is confirmed by provocative hormonal tests of pituitary reserve and computerized axial tomography or preferably, MR imaging.
Some patients with the initial picture clearly showing panhypopituitarism may recover partial pituitary function after cortisol replacement alone, such as thyroid or even gonadotropin function and the return of ovulation. It has been speculated that cortisol may have a permissive effect on other pituitary or hypothalamic hormones. Cases of spontaneous recovery and subsequent normal pregnancies have been reported as well.
A review of the outcome of pregnancies in women with Sheehans syndrome shows that in patients on adequate replacement therapy, the perinatal morbidity and mortality rates are not increased (87% live birth and 13% abortions). Treatment of the acute manifestations requires parenteral corticosteroids and intravenous fluids. Once the patient becomes stable the need for other hormonal replacement can be assessed by pituitary function testing.
Lymphocytic Hypophysitis
This disease entity has been described with increasing frequency in recent years. Most of the cases reported have been women during the last part of pregnancy or the postpartum period. Sheehan and Summers reported lymphocytic infiltration in the pituitary gland of some of their original cases, but the first report of a case of chronic lymphocytic hypophysitis as a distinct entity was not made until much later. It is believed to be an autoimmune disorder, and antibodies against pituitary cells have been described. In several cases there was also evidence of autoimmune involvement of other endocrine glands such as autoimmune thyroiditis and adrenalitis, and therefore these cases may be part of the autoimmune polyendocrine deficiency syndrome.
Almost all of the patients in the literature to date have been women in the last trimester of pregnancy or within 4 to 6 months after delivery; 65% were multiparous and 35% primiparous, and their mean age was 28 years. In more than half of the patients the initial symptoms were related to pressure from the expanding pituitary mimicking a pituitary tumor, including suprasellar extension with visual disturbances and headaches. Even though 78% of these cases had evidence of partial hypoadrenalism and 72% thyroid deficiency, the maternal and fetal survival was much better (7 of 12 survived) in these women than in those whose initial presentation was acute pituitary insufficiency, or if the symptoms went unrecognized until acute pituitary insufficiency occurred, at which point the mortality was very high. Other preceding symptoms noted included weight loss, weakness, hair loss, anemia and, in already postpartum women, oligo-or amenorrhea. Precipitating events were appendicitis, pneumonia, herpes labialis, flu-like illness, and in one case labor was the only obvious stress factor. The development of hypoglycemia along with the other symptoms of adrenal insufficiency was a poor prognostic sign. Unfortunately, the definitive diagnosis cannot be made currently unless pituitary tissue is obtained for histologic examination, and therefore the preoperative diagnosis of this potentially fatal but treatable disease is very difficult. The differential diagnosis during pregnancy includes, mainly, a pituitary tumor, or if the patient has diabetes, a pituitary infarction. After delivery, Sheehans syndrome has to be considered, particularly if there was excessive bleeding during labor.
The treatment of the acute event requires corticosteroid and intravenous fluid administration. Other hormonal deficiencies, if detected, can be replaced at a later date. A course of high-dose steroids has been recommended for those with visual field defects, but if there is no response surgery may be necessary. Spontaneous recovery has been observed as well. Fetal and maternal survival should improve with increased awareness of this disease entity and its symptoms.
Diabetes Mellitus
Nine cases of antepartum pituitary insufficiency in insulin-dependent diabetes have been reported. The mean maternal age was 28 years (range, 22-40), duration of diabetes was 7 years (range, 1 19) and the mean gestational age was 27 weeks (range, 12 36), with five of the nine cases occurring at 32 to 36 weeks. Three maternal and five fetal deaths occurred. Death has been attributed to pituitary infarction, but in most cases the duration of diabetes did not seem long enough to make vascular complications likely. The pituitary glands that were examined showed necrosis. Clinically the patients developed severe headaches, visual disturbances, decreased insulin requirements, and even hypoglycemia. The acute event was due to adrenal insufficiency. In the survivors, various other pituitary hormonal deficiencies requiring replacement were found.
Isolated Growth Hormone Deficiency
Several pregnancies have been reported in women of short stature caused by isolated GH deficiency. Placental function, fetal growth, pregnancy, and delivery were normal.
Replacement Therapy
Patients with known pituitary insufficiency but already on adequate replacement do not usually require a dosage change in their daily glucocorticoid replacement, which is 20 to 25 mg/m, or 30 to 37.5 mg of hydrocortisone or an equivalent steroid (Table 2). Two thirds (e.g., 20-25 mg) of the daily dosage is given in the morning, and one third (10-12 mg) in the late afternoon. At times of stress, such as with an infection or during labor and delivery, higher doses must be given (up to 300 mg of hydrocortisone or an equivalent steroid per day, given in divided dosages: 100 mg every 8 hours) with gradual tapering to the maintenance dose while the stress subsides. Mineralocorticoid replacement is not necessary if the deficit involves ACTH deficiency alone. Many pregnant women on thyroxine replacement, however, do need higher dosages during pregnancy, and therefore thyroid function tests must be performed at regular intervals (at least each trimester). When the hypothyroidism is because of pituitary or hypothalamic disease, the TSH cannot be used as a gauge for thyroxine replacement. In these patients we recommend keeping the total serum thyroxine in the upper or slightly above normal level, and the free thyroxine index in the middle to upper normal range.
Relative Biologic Effects Of Corticosteroids
| Preparations | |||
| Steroid | Anti-inflammatory Effect | Carbohydrate Effect | Sodium Retention |
| Cortisone | 1 | 1 | 1 |
| Hydrocortisone or Cortisol | 1.25 | 1.25 | 1.25 |
| Prednisone | 5 | 5 | 0 |
| Prednisolone | 5 | 5 | 0 |
| 6n-Methylprednisolone (Medrol) | 6 | 6 | 0 |
| Dexamethasone (Decadron) | 30 | 30 | 0 |
| Betamethasone (Celestone) | 30 | 25 | 0 |
| Fludrocortisone (Florinef) | 10 | 10 | 400 |
| Deoxycorticosterone (Doca) | 0 | 0.01 | 40 |
Pituitary Tumors
Prolactinomas
Prolactin secreting pituitary adenomas are the most commonly encountered pituitary tumors in women of childbearing age; they have also become the most frequent pituitary tumors found during pregnancy because of the availability of effective treatments to restore fertility in these women. With the advent of PRL radioimmunoassays and newer radiologic techniques (MR imaging) to detect pituitary abnormalities, the frequency of hyperprolactinemia and infertility caused by pituitary tumors has seen a marked increase. There was speculation that the widespread use of oral contraceptives since the 1960s might have had a role in the development of these tumors, but the Pituitary Adenoma Study Group concluded that no evidence linked estrogen use and prolactinomas in most of the studies reported to date.
Prolactinomas are diagnosed when the PRL becomes high enough to cause galactorrhea or oligoamenorrhea. Sometimes a tumor is not found and these cases are labeled as idiopathic hyperprolactinemia. This diagnosis is made only after an exhaustive work-up has excluded a pituitary tumor or any other cause of hyperprolactinemia, such as drug-induced, renal failure, cirrhosis of the liver, or several endocrine disorders, including primary hypothyroidism, Cushings syndrome, and acromegaly.
Pituitary tumors are divided, according to their size, into micro (less than 10 mm) and macroadenomas; they are further classified according to suprasellar extension or invasion of other adjacent structures. Once the diagnosis is confirmed and the patient desires to become pregnant, several therapeutic modalities are available.
Pituitary radiation had a low curative rate in the earlier reports. Recent studies show more favorable results, but the reduction of PRL level is very slow and hypopituitarism still seems to be a common late sequela. It was formerly used, before pregnancy, with the intention of preventing enlargement of the tumor during gestation, although it did not seem to do so in some cases. Radiation therapy has been more commonly used in patients with residual disease despite surgery. Other forms of radiation, such as implantation of yttrium-90 in the pituitary gland, also have been used in the past.
Surgical treatment, mainly transphenoidal removal of the tumor, has been effective in reducing or normalizing PRL levels and in restoring fertility, particularly in patients with smaller tumors. Long-term (4-5 years) studies have shown, however, recurrence of hyperprolactinemia requiring additional therapy in 25% to 40% of the patients. Despite the expense and potential morbidity, transphenoidal adenomectomy is an accepted form of therapy and may be the more reasonable option for those unable to tolerate bromocriptine or those who do not want to take medication on a long-term basis. The best results are consistently reported in patients with small tumors. Macroprolactinomas are easier to diagnose but surgical cures are obtained much less frequently; in these cases surgical decompression may facilitate postoperative medical therapy and perhaps offer some protection against enlargement during a subsequent pregnancy.
Medical therapy with bromocriptine, a long-acting dopamine receptoragonist, has been very effective in restoring ovulation in 80% to 90% of hyperprotaclinemic women with microadenomas. Most women respond to doses of 2.5 to 7.5 mg/d, although occasionally higher doses are needed. Besides normalizing PRL levels, therapy with bromocriptine has been shown to have a low risk of tumor enlargement and in some cases it may even be able to reduce tumor size. Early reports of successful pregnancies after bromocriptine therapy were encouraging and, since then, more have followed to confirm the initial impression. If conception occurs, bromocriptine is discontinued and the patient is followed closely. It has been recently reported that even in patients with large tumors, including those with suprasellar extension, bromocriptine therapy could be safely withdrawn during pregnancy; others advise to continue therapy without waiting for complications to occur.
Serum PRL levels during pregnancy in women with prolactinomas are not, in general, predictable of tumor growth, and serial measurements are not recommended. In patients with microadenomas the risk of symptomatic tumor growth during pregnancy is very low (1% – 2%) and therefore serial visual field examinations or MR pituitary imaging is not recommended unless symptoms appear. If only computed tomography is available the abdomen should be shielded. If sever headaches occur, even in the absence of visual defects, an MR imaging of the pituitary gland should be considered, and it should be mandatory if visual field defects are present. If tumor enlargement is detected, therapy with bromocriptine is initiated immediately and visual field examinations are performed daily. If no rapid clinical response occurs despite increasing doses of bromocriptine (up to 20 mg/d), glucocorticoid treatment is initiated (e.g., dexamethasone 4 mg every 6 hours). If both treatments fail, surgery should be strongly considered.
In Untreated macroadenomas the risk of tumor growth during pregnancy is higher (15%-25%) but still low (4%) in women treated (bromocriptine, surgery, radiation, single or in combination) before conception. In these women, monthly visual field examinations are recommended, as well as MR imaging if there is suspicion of tumor enlargement. Besides headaches and visual disturbances, diabetes insipidus or pituitary infarction may occur rarely. The first trimester is thought to be when complications are more likely to develop. Surgery during pregnancy has been reported in a few cases, but therapy with bromocriptine generally has been effective, with an occasional failure. The continuation of bromocriptine therapy is recommended until after delivery when tumor growth occurs during pregnancy. Labor and delivery usually are uncomplicated, but in patients who experience tumor growth it may be advisable to shorten the duration of the second stage to avoid the elevation of intracranial pressure that may occur while pushing.
Few complications of bromocriptine therapy during pregnancy have been reported. Early reports of cervical incompetence have not been subsequently confirmed. In 1410 pregnancies where bromocriptine was used, including over 100 treated throughout gestation, the following rates were reported: abortion, 11.1%; multiple gestation, 1.2%; prematurity, 10.1%; and congenital anomaly, 3.5% (2.5% minor, 1% major). These results are similar to those reported for patients in a general infertility population when pregnancy finally occurs. No mental or physical abnormalities have been reported in exposed children aged up to 5 years.
Breast-feeding is not contraindicated in women with prolactinomas. Spontaneous remissions have been observed after delivery, particularly in those with small tumors and PRL levels of 60 ng/mL or less, which has led to the speculation that pregnancy might be even beneficial instead of dangerous in some cases.
The risks versus benefits of the various treatment options should be carefully discussed with the patient before pregnancy. The choice of treatments for patients who desire to conceive is summarized in table 3.
Prepregnancy Management of Hyperprolactinemia
| Condition | Management | Pregnancy Follow-Up |
| No tumor | Bromocriptine* | No special tests |
| Microadenoma | Bromocriptine* or surgery | Visual fields/MR imaging only if symptomatic |
| Macroadenoma | Surgery Surgery + Bromocriptine Radiation + Bromocriptine Bromocritine* alone? |
Monthly visual field examinations MR imaging if symptomatic |
*Therapy for 1 y before conception?
The Surgical Treatment of Prolactinomas
Predictors of Outcome and Long-Term Results
The February, l999 issue of the journal Neurosurgery contains a report by Tyrrell and colleagues from the University of California in San Francisco (UCSF) in which early outcomes and long-term results are analyzed in a large series of patients with prolactinomas following transsphenoidal microsurgery. Two groups of patients treated in different periods of time were followed, and outcomes including complications were evaluated to provide information on the benefits and risks of microsurgery in the management of prolactinomas. The author, J. Blake Tyrrell, is a Professor of Medicine and Head of the Section of Endocrinology at UCSF. All patients were operated on by Charles B. Wilson, Professor of Neurosurgery at UCSF. The statistical analysis was performed by Kathleen R. Lamborn, also at UCSF.
Data were reviewed for two groups of female patients: 121 patients treated surgically between 1976 and 1979 (Group 1) and 98 patients treated between 1988 and 1992( Group 2). Males were excluded because the numbers of males in these two periods were too few. The two time periods were chosen to determine if the later series had better outcomes based on greater experience and improved imaging, and the earlier series was chosen to obtain long-term outcomes in a series of patients treated at least fifteen years earlier.
Definitions
Normal prolactin (PRL) values were defined as 20ng/ml or less, or when a particular laboratory had higher upper limits of normal values, the laboratory’s definition of normal was accepted as normal. The postoperative PRL value was the lowest value documented during the first 7 days after surgery. Postoperative remission was defined as a PRL value of 20ng/ml or less measured within this interval. The duration of follow-up was the time in years to the date of the last available PRL value, and patients classified as experiencing continued remission exhibited no clinical symptoms of hyperprolactinemia, had undergone no additional therapy and demonstrated normal PRL values. Patients initially experiencing remission were classified as having the recurrent disease at the date of the first documented occurrence of any of the following: a PRL value of >20ng/ml or higher than the normal range for the laboratory, recurrence of amenorrhea or galactorrhea, or further treatment. Technical recurrence was defined as an elevated PRL value without symptoms after an initial remission and thereafter normal PRL values with no additional therapy. Continued clinical remission with hyperprolactinemia was defined as a slight elevation of PRL (22-33 ng/ml) with no symptoms and no requirement for additional therapy.
Results
The two groups were very similar in all respects although the earlier patients were more likely to have had amenorrhea for longer periods before treatment, and the later series of patients were more likely (61%) to have received bromocriptine before operation. Grade and stage of the adenomas was similar. Combining the two groups, 72% in the series achieved initial surgical remission: (91% for intrasellar microadenomas and 88% for intrasellar macroadenomas). However, there was a significant difference between women in Group 1 and Group 2 in the median postoperative PRL value (6 versus 2 ng/ml respectively).
Of the 132 patients who experienced initial remission, at the last follow-up evaluation, 89% remained in remission and 11% had experienced recurrence. Reflecting both a shorter period of follow-up and greater experience, 97% of patients in Group 2 remained in clinical remission at the last follow-up (median follow-up of 3.2 years) and only 2 patients ( 3%) had experienced recurrence.
Univariate analysis of the time to recurrence revealed that the postoperative PRL value was the best predictor of continued remission, although older age and lower adenoma stage were also significant.
Complications were more frequent in Group 1 ( 7 complications), 6 of seven being related to a postoperative cerebrospinal fluid leak or hematoma requiring re-operation or treatment for meningitis. In Group 2 there were only two serious complications, a postoperative intrasellar hematoma that did not require re-operation, and a cerebrospinal fluid leak that was treated successfully with temporary insertion of a spinal drain without re-operation. No deaths have occurred in more than 1,000 operations for prolactinoma spanning a period that includes the patients in Groups 1 and 2.
Discussion
The results of this study confirm the immediate and long-term efficacy of transsphenoidal surgery in the treatment of women with prolactinomas. Initial remission was achieved in 92% of those with PRL values of <100ng/ml. Patients with preoperative values >200ng/ml and large or invasive adenomas achieved less favorable outcomes. There were no differences in remission rates for Groups 1 and 2, although postoperative PRL values were significantly lower in Group 2. The likelihood of recurrence is lower in patients with the lowest postoperative PRL levels (2ng/ml or less). Prior therapy with bromocriptine had no effect on immediate surgical outcomes. This series compares favorably with 31 previously reported series with initial remission being achieved for 91% of women with intrasellar microadenomas, 82% of all women with microadenomas, and 66% of women with macroadenomas.
The most controversial aspect of surgical therapy is the wide variation in reported rates of recurrent hyperprolactinemia, the figure of 50% being quoted commonly in quantifying the risk of recurrence. The patients in our Group 1 represent those with the longest follow-up periods reported to date for transsphenoiday microsurgery, and our results do not confirm previously reported high recurrence rates. In our Group 1, with a median follow-up period of 15.6 years ( mean 13.0 years), 82% of patients exhibited normal PRL values and one patient demonstrated mild recurrent hyperprolactinemia without symptoms at their latest evaluations. Therefore, 84% of these patients with initial remission continued in clinical remission, yielding a recurrence rate of 16%. Other series reporting comparably low recurrence rates had follow-up periods of 5 years or less.
Conclusion
Both medical therapy and transsphenoidal surgery have roles in the optimal treatment of PRL-secreting adenomas. Although treatment with dopamine agonists has the advantages of non-invasive therapy, transsphenoidal microsurgery can afford selected patients immediate normalization of PRL levels, without the need for continuing dependence on medication. However, patient selection must be done with the knowledge that those patients with the highest likelihood of long-term remission are those with PRL levels of 200ng/ml or less and a microadenoma or noninvasive macroadenoma. It is equally important to assess the skill and experience of the surgeon performing the procedure. Transsphenoidal microsurgery is an effective alternative to long-term medical therapy for selected patients with prolactimomas.
Available Now!

The Pituitary Patient Resource Guide Sixth Edition is now available! Be one of the first to have the most up-to-date information. The Pituitary Patient Resource Guide a one of a kind publication intended as an invaluable source of information not only for patients but also their families, physicians, and all health care providers. It contains information on symptoms, proper testing, how to get a diagnosis, and the treatment options that are available. It also includes Pituitary Network Association's patient resource listings for expert medical care.

Xeris Pharmaceuticals is valued member of the PNA










